Jun 7, 2011
Pandemic H1N1 hit children with asthma harder than seasonal flu
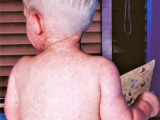
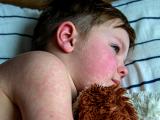
Almost half of all children hospitalized for 2009 H1N1 pandemic flu had asthma, compared with a third of kids hospitalized each year for seasonal flu, according to a study yesterday in Pediatrics. Researchers from the US Centers for Disease Control and Prevention and 10 states found that 701 (32%) of 2,165 children hospitalized during the 2003 through 2009 influenza seasons had asthma, compared with 733 (44%) of 1,660 who had 2009 H1N1 flu. Hospitalized children with asthma who had pandemic 2009 H1N1 were more likely to require intensive care (22% vs 16%, P = .01) and have pneumonia (46% vs 40%, P = .04) compared with seasonal-flu patients. However, rates of respiratory failure (5%) and death (1%) were the same in both groups. Also, more children with influenza A, whether seasonal or pandemic, experienced asthma exacerbations compared with those who had influenza B (51% vs 29%, P < .01). The authors say the findings underscore the importance of vaccination in children with asthma.
Jun 6 Pediatrics abstract
Australian study shows good safety profile in kids for this year's flu vaccine
After administering more than 2,000 doses of influenza vaccine to children, scientists in Western Australia (WA) are reporting low instances of serious adverse events, according to a letter in the Medical Journal of Australia. From Mar 15 to Apr 29, 2,227 doses of flu shots were administered to children under five years old in WA: 2,130 doses of Vaxigrip (Sanofi Pasteur) and 97 doses of Influvac (Solvay). Of those, adverse events were reported to the Western Australian Vaccine Safety Surveillance: two cases of fever (yet below 39.5°C, or 103°F), one of vomiting and diarrhea, and one of fever and convulsions 4 days after vaccination (in a child who had a respiratory tract infection). All four children had been administered other vaccines with flu vaccine. In a safety study, 144 children received a flu shot in same time period. Adverse events after vaccination were reported in 10 children (7%), 2 of whom received other vaccines in addition to flu vaccine. All 10 children had fever reported, and one child had a temperature greater than 39.5°C. Two children experienced vomiting, but no convulsions were reported and no events required medical care. This compares with an incidence of febrile convulsions after vaccination with Fluvax and Fluvax Junior (both from CSL Biotherapies) last year of 4.4 per 1,000 doses. Fluvax will not be administered to children younger than 5 years in Australia this year.
Jun 6 Med J Aust letter
Vaccination urged after measles cases in Quebec top 250
After witnessing more than 200 measles cases since May 1, Quebec's director of public health, Alain Poirier, is urging people to get vaccinated against the disease, according to a Canadian Press (CP) article today. The province has recorded 208 cases since May 1 and 254 in 2011 in several regions. Cases in April seem to have originated in people returning from France, but since then cases "appear to be of local origin," the story said. "We usually see one or two cases of measles every year," Poirier said. "Episodic cases of this contagious illness, however, justify continued vaccination efforts from the point of view of prevention and we invite people to use this method to protect themselves."
Jun 7 CP story
PATH announces collaboration toward 2nd-generation malaria vaccine
The PATH Malaria Vaccine Initiative (MVI) announced today it has entered into a partnership with Dutch pharmaceutical company Crucell and London-based GlaxoSmithKline (GSK) to develop a second-generation malaria vaccine. The collaboration will combine two vaccine approaches to improve the efficacy of GSK's first-generation RTS,S vaccine candidate, according to an MVI press release. The effort will entail administering one dose of Crucell's weakened recombinant adenovirus Ad35.CS.01 malaria vaccine, followed by two doses of GSK's RTS,S vaccine in a phase 1/2a clinical trial expected to begin later this year. This would be the first test in humans of a "heterologous prime-boost" approach against malaria. MVI Director Dr. Christian Loucq said in the release, "This new collaboration, though in the early stages, gives us the opportunity to test an approach with the potential to substantially increase efficacy and move us closer to the internationally agreed upon goal of an 80% effective second-generation vaccine by 2025." Malaria kills close to 800,000 people annually, according to MVI. PATH is an international nonprofit global health organization based in Seattle.
Jun 7 MVI press release