For over two decades, Lynn Cole was in a protracted battle with bacteria and her own immune system.
Diagnosed as having the autoimmune disease Sjogren's syndrome in 1999, Cole suffered from pulmonary fibrosis, was oxygen dependent and highly susceptible to pneumonia, and frequently needed antibiotics for recurrent lung and urinary tract infections. Her daughter, Mya, was there for all of it.
"Most of my childhood was doctor's appointments, inpatient hospital stays, treatments…all that kind of stuff," Mya Cole told CIDRAP News.
But around 2010, Lynn Cole began to have recurrent bloodstream infections caused by the bacterium Enterococcus faecium. From 2013 to 2020, she underwent several hospitalizations at University of Pittsburgh Medical Center (UPMC) for E faecium bloodstream infections and received multiple courses of intravenous antibiotics. At some point in her complex medical history, the bacterium had colonized her gut and become the source of the recurrent infections.
Over that period, Cole, whose case was described in a recent report published in the journal mBio, would typically be sent home with a PICC (peripherally inserted central catheter) line to continue the antibiotic treatment. But within a few days of finishing the antibiotics and removing the PICC line, Cole's blood cultures would be positive for E faecium again.
"We just continued that cycle over and over again, which was frustrating," Mya Cole said.
The cycle continued, with increased frequency, into late 2020, when Cole experienced 26 days of persistent E faecium bacteremia despite treatment with multiple antibiotics that showed in vitro activity against the bacteria.
At that point Cole's treatment team suggested bacteriophages—bacteria-killing viruses—as a potential solution. Cole, after conferring with Mya and her partner, Tina Melotti, said yes.
"We did a little research, and then we talked as a family and agreed that if it could give us a chance, we would try it," Mya said.
A phage cocktail suppresses the infection
To find a phage that might work for Lynn Cole's infection, her doctors turned to researchers at UPMC's Van Tyne lab, which studies how bacteria evolve to resist antibiotics and develops new approaches to treat resistant infections. After receiving the request from Cole's doctors in June 2020, when it had become clear that antibiotics were not going to solve the problem, the lab set out to find a phage that matched the strain of E faecium that was causing the recurrent infections.
Phages aren't hard to find, because they're one of the most abundant organisms on the planet. They can be found in soil, plants, sewage water, and even in the human body. But unlike antibiotics, which work against a narrow or broad spectrum of bacteria, phages have to match the exact strain of bacterium they are targeting to have an effect. That requires testing isolates from a patient's infection to find a match.
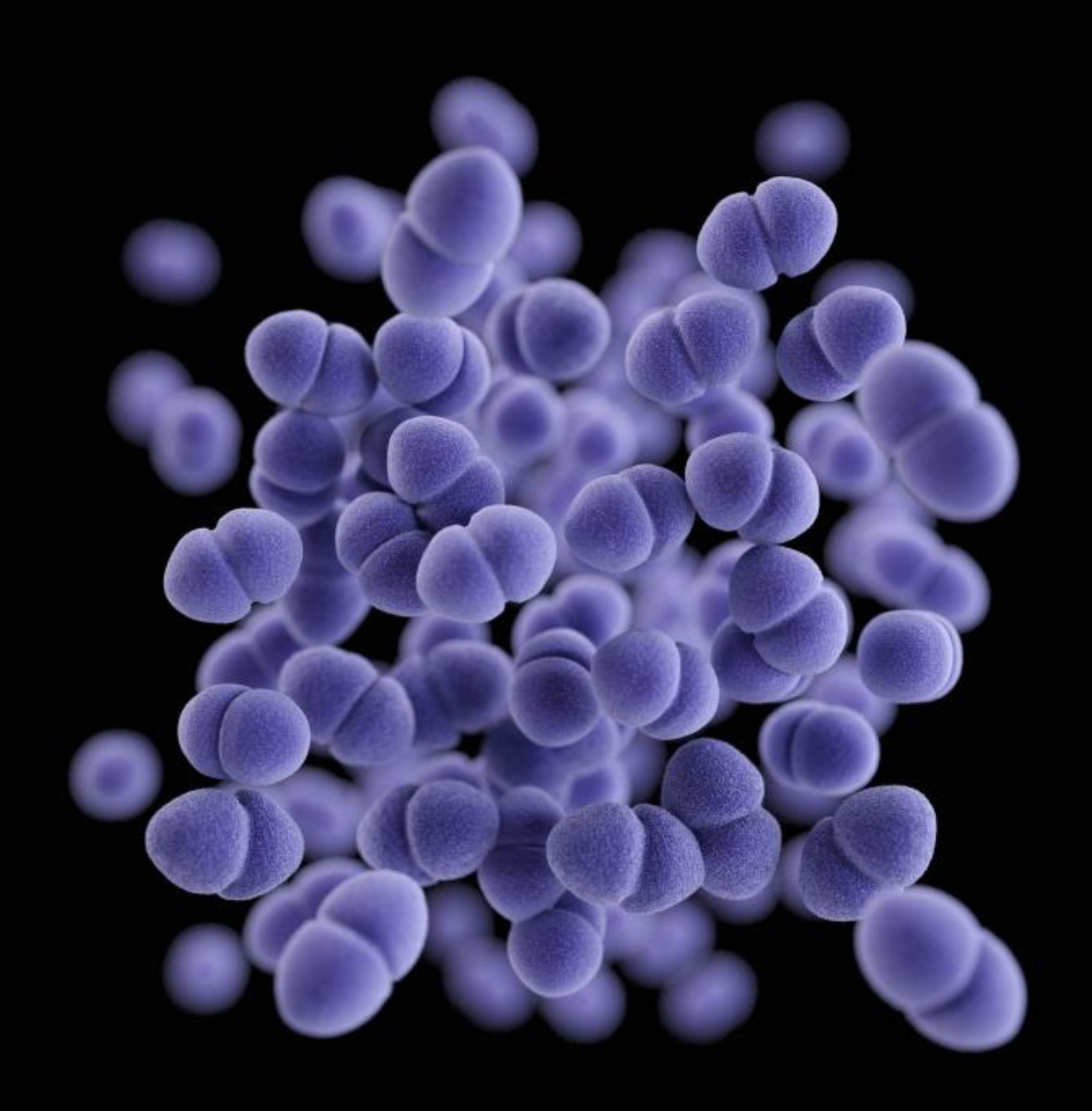
Once a match is found, the identified phage then has to be grown, purified, and prepared for use in a patient. And that's only part of the lengthy process. Because phages are not approved for use in the United States, an Emergency Investigational New Drug (eIND) application for each individual case has to submitted to the US Food and Drug Administration to get the go-ahead.
Ultimately, scientists at the University of Colorado found a phage—9184—that had activity against isolates collected from Cole's infection and sent it the Van Tyne lab, where it was propagated and purified. In December 2020, after spending 20 days in the intensive care unit, Cole began receiving three daily doses of the phage in combination with systemic antibiotics.
"And then, within 24 hours, the blood cultures were clear for the first time that month," said Madison Stellfox, MD, PhD, a member of the Van Tyne lab and co-author of the case report.
After being sent home from the hospital, Cole continued receiving antibiotics and the phage therapy through the PICC line under the supervision of Mya and Tina, both of whom work in healthcare. After a few breakthrough infections that were able to be managed at home, Stellfox and her colleagues added another phage—Hi3—to the treatment regimen.