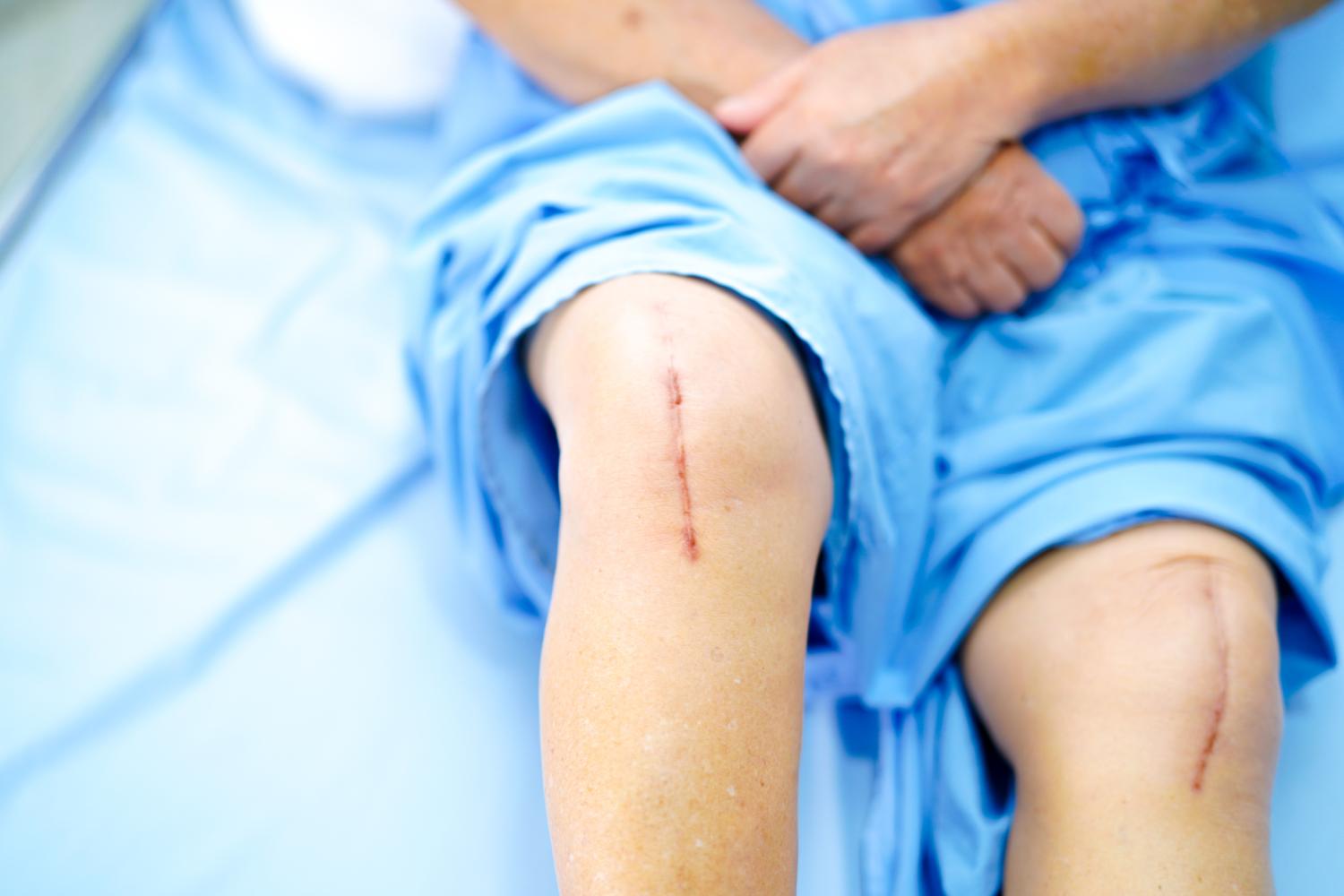
The results of a randomized trial conducted in Australia indicate that for joint replacement surgery, more antibiotics aren't better—and may be even worse.
The trial, which involved more than 4,000 patients undergoing knee, hip, and shoulder replacement, found that administering vancomycin in addition to cefazolin at the time of surgery did not reduce surgical site infections (SSIs) compared with placebo. In some cases, in fact, it may have led to more infections, as well as more adverse events.
The investigators say the findings, published today in the New England Journal of Medicine, are significant for antibiotic stewardship because joint replacements are increasing as the population ages. In the United States alone, they note, more than 2.7 million joint replacement procedures are expected to be performed annually by 2030.
"Given the number of joint replacements performed in Australia and globally, our trial has answered the important [question] about whether more antibiotics are better for our patients having joint replacement surgery: with the definitive answer being 'no,'" lead trial investigator Trisha Peel, MBBS, PhD, of Monash University Central Clinical School, said in a university press release.
More infections with vancomycin
For the Australian Surgical Antibiotic Prophylaxis (ASAP) trial, Peel and her colleagues randomly assigned 4,239 patients without known methicillin-resistant Staphylococcus aureus (MRSA) colonization who were undergoing arthroplasty to receive 2 grams of prophylactic cefazolin with 1.5 grams of vancomycin or cefazolin with placebo. The primary outcome was SSIs within 90 days.
Under current guidelines, patients undergoing joint replacement receive a second-generation cephalosporin, such as cefazolin, at the time of surgery to prevent SSIs, which can result in high morbidity and mortality. Many Australian hospitals, however, have added vancomycin to this regimen to broaden the spectrum against pathogens like MRSA, which is being increasingly reported in post-arthroplasty SSIs. The trial aimed to assess the efficacy of this practice.
Among the 4,113 patients in the modified intention-to-treat population (2,233 undergoing knee replacement, 1,850 undergoing hip replacement, and 50 undergoing shoulder replacement), SSIs occurred in 163 patients (4.0%) overall, and most were superficial. SSIs occurred in 91 of 2,044 (4.5%) of patients in the vancomycin group and 72 of 2,069 (3.5%) in the placebo group (relative risk [RR], 1.28; 95% confidence interval [CI], 0.94 to 1.73).
The increased risk of SSI was most noticeable among the patients undergoing knee replacement, with SSIs occurring in 5.7% of patients the vancomycin group and 3.7% in the placebo group (RR, 1.52; 95% CI, 1.04 to 2.23). Among patients undergoing hip arthroplasty, SSIs occurred in 3.0% of patients in the vancomycin group and 3.1% in the placebo group (RR, 0.98; 95% CI, 0.59 to 1.63). In shoulder arthroplasty, the only SSI occurred in the placebo group.
Given the number of joint replacements performed in Australia and globally, our trial has answered the important [question] about whether more antibiotics are better for our patients having joint replacement surgery: with the definitive answer being 'no.'
Among the 4,040 patients included in the safety analysis, adverse events occurred in 1.7% of patients in the vancomycin group and 1.7% in the placebo group. Vancomycin prophylaxis was associated with an increased risk of hypersensitivity reactions (RR, 2.20; 95% CI, 1.08 to 4.49) and a decreased risk of acute kidney injury (RR, 0.57; 95% CI, 0.39 to 0.83) compared with placebo.
"The addition of vancomycin to cefazolin prophylaxis was not superior to placebo for the prevention of surgical-site infections in arthroplasty among patients without known MRSA colonization," the study authors concluded.
The authors say previous observational studies that have found a reduction of SSIs with the addition of vancomycin often involved patients with a high incidence of MRSA and that other infection-prevention strategies, such as decolonization, may have contributed to fewer infections.
They also note that while current Australian guidelines recommend the addition of vancomycin in arthroplasty patients with known MRSA infection or colonization, the trial excluded such patients. "Thus, our results cannot be extrapolated to these patients, " they wrote.
Peel said the study highlights the importance of large, multicenter randomized trials. "A lot of things seem to make sense, but we don't really know for sure until they are tested in a clinical trial," she said. "This is one of those cases."