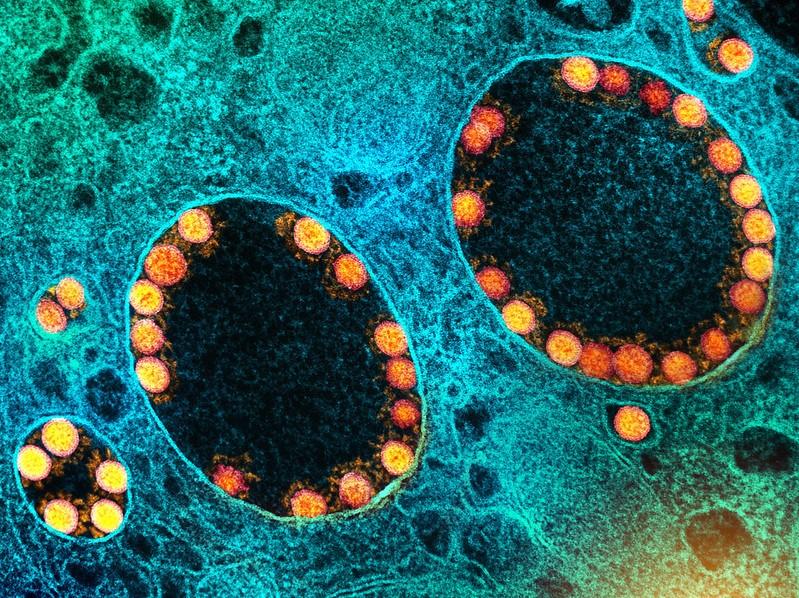
Today President Joe Biden addressed the nation on the Omicron (B.1.1.529) variant, a concerning strain of the COVID-19 virus first identified in South Africa late last week. Biden said the variant was a cause for concern—not panic—and cautioned that although newly placed travel restrictions will buy the county some time, the variant will likely soon be in the United States.
"Restricting travel gives us time to take more actions, move quicker to make sure people understand they have to get the vaccine, you have to get the shot, you have to get the booster," Biden said.
The country is limiting travelers from South Africa, Botswana, Zimbabwe, Namibia, Lesotho, Eswatini, Mozambique, and Malawi, beginning today, CNN reports. The restrictions do not apply to US citizens and lawful US permanent residents.
Biden said his top advisors, including Anthony Fauci, MD, believe the current vaccines in use in the United States will offer some protection against the variant strain, but the administration is working with scientists from Pfizer-BioNTech, Moderna, and Johnson & Johnson to develop contingency plans if needed.
Throughout the address, Biden assured reporters that shut downs and lockdowns will not be used, because the country has such ample vaccine supply, and indoor mask requirements work to stop the spread of the virus.
Vaccine makers react to Omicron
As news of the new variant spread over the holiday weekend, chief executives at Moderna and Pfizer said they could quickly reformulate existing vaccines to address the variant if needed.
In a statement to NPR, Moderna said it has been studying two booster vaccines that are designed to anticipate mutations like those found in the Omicron variant, and will ramp up efforts to make a booster that specifically targets Omicron. The company is also considering doubling the dose of the current booster from 50 to 100 micrograms.
Pfizer CEO Albert Bourla told CNBC that the company could produce a new vaccine against the Omicron variant within 100 days.
Both executives said they would need another 2 weeks to determine how the Omicron variant stands against current vaccine formulations.
Currently all American adults who completed their primary vaccine series 6 months ago are eligible for booster doses of vaccine. Both Biden and Fauci urged all who are able to do so to be boosted as soon as possible.
CDC urges boosters
The Centers for Disease Control and Prevention Center (CDC) also issued a statement today strongly recommending COVID vaccine boosters for all US adults.
"Everyone ages 18 and older should get a booster shot either when they are 6 months after their initial Pfizer or Moderna series or 2 months after their initial J&J vaccine," CDC Director Rochelle Walensky, MD, MPH, said in a media statement.
"I strongly encourage the 47 million adults who are not yet vaccinated to get vaccinated as soon as possible and to vaccinate the children and teens in their families as well because strong immunity will likely prevent serious illness.
"I also want to encourage people to get a COVID-19 test if they are sick. Increased testing will help us identify Omicron quickly."
The CDC COVID Data Tracker shows that 59.1% of Americans are fully vaccinated against COVID-19, 69.7% have received at least one dose of vaccine, and 19.1% of fully vaccinated Americans have received a booster dose.
FDA advisers to consider COVID pill
Tomorrow the Food and Drug Administration's (FDA's) antimicrobial advisory committee meets to discuss an emergency use authorization for Merck's molnupiravir oral treatment.
Late last week the company posted an update on clinical trial findings, which suggest the drug had less of an impact than initial results showed—a 30% risk reduction in hospitalization and death versus the 50% they reported earlier. Findings are based on 1,433 COVID-19 patients.
The FDA is signaling that the pill seems to be effective, but it wants extra outside input on whether it is safe for pregnant woman, according to the Associated Press. The FDA said animal studies have shown potential toxicity to fetuses, as well as birth defects.
Other US developments
- The United States reported 28,213 new COVID-19 cases yesterday and 103 deaths, according to the Johns Hopkins COVID-19 tracker. The 14-day average of new daily cases is 83,365, with 961 daily deaths, according to the New York Times tracker.
- New York Gov. Kathy Hochul on Nov 26 declared a state of emergency in response to a surge of coronavirus infections in the state and the threat of the Omicron variant, according to the Washington Post.
- Thousands of American service members are facing disciplinary action, including dismissal, if they are not fully vaccinated against COVID-19 by today, CBS News reports.