Two new US studies suggest that the bivalent (two-strain) Pfizer/BioNTech mRNA COVID-19 vaccine offered children and adolescents good protection against severe outcomes such as hospitalization during Delta and Omicron predominance and that vaccine-induced antibodies can neutralize Omicron BA.2.86 but that the subvariant has features linked to severe symptoms.
The bivalent vaccine was replaced by a monovalent (single-strain) version in fall 2023.
85% to 91% effective against ICU stays
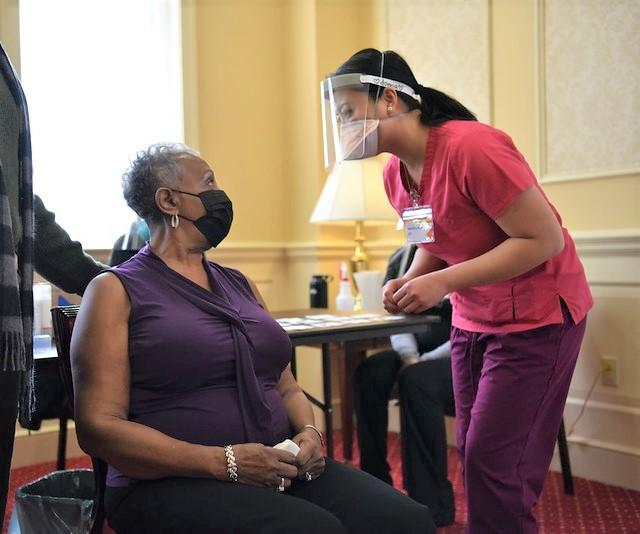
In an observational comparative-effectiveness study, researchers from the University of Pennsylvania and Children's Hospital of Philadelphia (CHOP) analyzed data on vaccine effectiveness (VE) against infection and severe disease from 250,000 never-infected COVID-19 patients at pediatric health systems participating in the national PEDSnet collaboration. About half of participants had received at least one dose of the Pfizer vaccine during the Delta and Omicron waves that began in mid-2021 and 2022, respectively.
The results were published today in the Annals of Internal Medicine.
Study participants were included in one of three cohorts: adolescents aged 12 to 20 years during the Delta wave (77,392 participants; 58.2% vaccinated) and children aged 5 to 11 years (111,539 participants; 45.2% vaccinated) and adolescents aged 12 to 20 (56,080 participants; 38.0% vaccinated) during the Omicron period.
During Delta, estimated VE was 98.4% against infection among adolescents, with no significant waning after the first dose and no significant difference in cardiac complications between vaccinated and unvaccinated groups. During Omicron, estimated VE against infection in children was 74.3%. Higher VEs were seen against moderate or severe illness (75.5%) and intensive care unit (ICU) admission (84.9%).
Although the pandemic has been declared over, the risk of COVID-19 is present throughout U.S. communities.
In adolescents, estimated VE against Omicron infection was 85.5% (84.8% against moderate or severe COVID-19 and 91.5% against ICU admission). VE against Omicron waned 4 months after dose one and then leveled off. Vaccinated participants were at lower risk for cardiac complications during this period.
"Our assessment of vaccine effectiveness across diverse outcomes underscores the vaccine's pivotal role in reducing SARS-CoV-2 transmission, minimizing COVID-19–related sick leaves, and alleviating economic burdens during the pandemic," the study authors concluded.
In a UPenn news release, co-senior author Christopher Forrest, MD, PhD, of UPenn and CHOP, noted that COVID-19 vaccine clinical trials enrolled children and adolescents last. "Although the pandemic has been declared over, the risk of COVID-19 is present throughout U.S. communities," he said. "Thus, more information is needed on effectiveness of vaccination delivered to children and adolescents during more recent time periods."
Vaccine-induced antibodies neutralize BA.2.86
For a cell-culture study published yesterday in Cell, Ohio State University researchers analyzed neutralizing antibodies in serum samples from healthcare providers who received three monovalent vaccine doses or two monovalent doses followed by a bivalent booster and from first responders infected with COVID-19 during the Omicron XBB.1.5 wave.
They compared the ability of neutralizing antibodies to prevent infection by BA.2.86, the FLip XBB-derived variant, the wild-type virus, and several other variants. BA.2.86 is the ancestor of the currently dominant Omicron JN.1 variant and has more than 30 spike-protein mutations than its close Omicron relatives.
If you have less severe disease, the antibodies generated by infection are low—almost 10-fold lower than vaccine-induced antibodies.
Bivalent vaccine-induced serum antibodies produced in healthcare providers were more efficient at neutralizing BA.2.86 than at neutralizing other Omicron variants, including XBB.1.5, which the authors said explains why BA.2.86 didn't cause a huge surge. But the three monovalent vaccines and previous XBB.1.5 infection were only marginally effective in preventing BA.2.86 infection.
Also, they found that BA.2.86 can infect the cells that line the lower lungs and fuse with the host cell membrane more efficiently, two features tied to severe COVID-19 symptoms.
"People who have had a COVID-19 infection should remember that omicron variants are less virulent compared to prior variants such as delta, meaning they don't make most people very sick," senior author Shan-Lu Liu, MD, PhD, of Ohio State, said in a university news release. "If you have less severe disease, the antibodies generated by infection are low—almost 10-fold lower than vaccine-induced antibodies. That is why you cannot rely on natural infection alone for immunity."
Because the bivalent COVID-19 vaccine is less effective against BA.2.86 than the currently available monovalent version, Liu advised getting a booster.