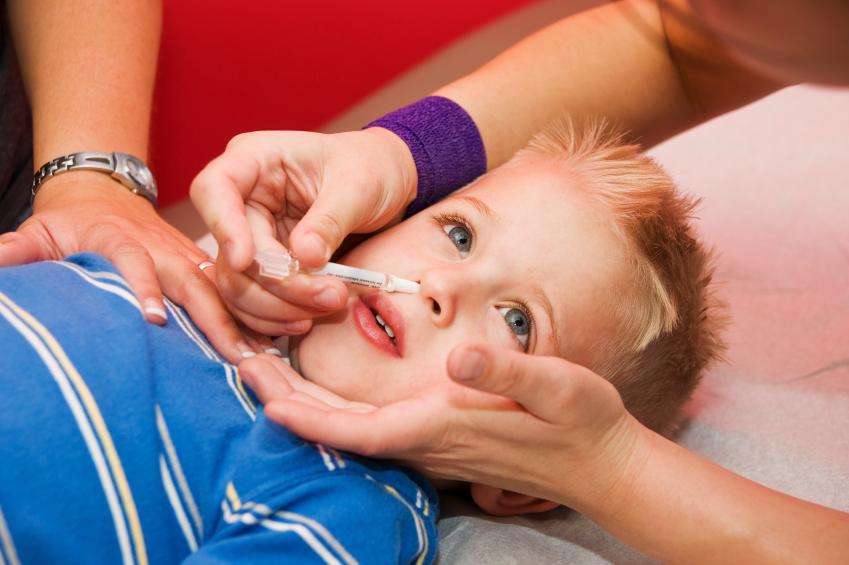
Federal vaccine advisors today, in a rare move, made a preferential recommendation for a flu vaccine, giving the nod to the nasal-spray vaccine over injected flu vaccines for healthy children ages 2 to 8 years old, based on studies that suggest the inhaled version has an efficacy edge.
The vote of the Advisory Committee on Immunization Practices (ACIP), an expert group that advises the US Centers for Disease Control and Prevention (CDC), followed several months of analysis by a working group that compared the two vaccines and offered its formal recommendation today.
The step follows similar moves by other developed nations, including Canada, the United Kingdom, Germany, and Israel. ACIP's vote was unanimous.
Over the past few years, several different flu vaccine formulations have entered the US market, and ACIP has rarely recommended one over another, except for in 2013 when it recommended a newly approved recombinant flu vaccine—Protein Science's FluBlok—for adults age 18 through 49 who have egg allergies, regardless of severity.
Experts compare LAIV and IIV
In its presentations today before the vote, working group members pointed to research that showing both live attenuated flu vaccines (LAIV) and inactivated flu vaccines (IIV) are safe and effective, with several studies suggesting that LAIV may have advantages for children, especially younger ones.
Ruth Karron, MD, who chairs the ACIP influenza working group, said after months of discussion about the evidence, the working group characterized the quality of evidence supporting the preferential recommendation as moderate to high.
In 2011, a meta-analysis of randomized controlled trials on flu vaccines concluded that LAIV had an efficacy of 83% in children 6 months to 7 years old, but that the trials didn't support efficacy in older children or adults. That study didn't find any randomized trials that supported the efficacy of trivalent IIV in children ages 2 to 17.
Some of the other countries that have a preferential recommendation for LAIV have a broader age range than what ACIP recommended. For example, the United Kingdom recommends it for children 2 to 18 years, and Canada and Israel's recommendation covers kids ages 2 to 17. Germany, however, recommends LAIV for a younger age range, for kids ages 2 to 6.
Canada updated its recommendation in November 2013 to note that the LAIV advantage is clear in children ages 2 to 6 years old but is less clear in older children.
Recommendation and caveats
ACIP's recommendation emphasizes that both LAIV and IIV are safe and effective. It specifies that while clinicians should administer LAIV if both it and IIV are on hand, they should not delay vaccination if LAIV is not immediately available.
The recommendation states, however, that LAIV shouldn't be used in kids who have had wheezing or asthma in the past 12 months, in those who have egg allergies, in those who have allergic reactions to vaccines or their components, or in kids who are on aspirin or immunosuppressive therapy.
In discussions before the vote, some members and representatives from other health groups aired concerns about the recommendation. Some worried that parents might perceive that their children would be at a disadvantage if they received IIV instead of LAIV.
Jeffrey Duchin, MD, a member of ACIP's influenza working group, said the team has tried to emphasize that both types of vaccines are safe and effective, but when possible, LAIV is preferable. "Not getting it is not a major disadvantage," he said.
Other concerns were the slightly higher cost of LAIV and the impact the ACIP's recommendation might have on immunization plans that are already under way.
Philip Hosbach, vice president for immunization policy for Sanofi Pasteur, one of the companies that makes IIV for the US market, said the recommendation's timing might impinge on some vaccine makers, which are already producing vaccine for the upcoming flu season. He said the move might prompt clinicians to cancel their orders and have a negative financial impact on stakeholders, including clinicians.
Though ACIP's recommendations aren't binding, the CDC generally adopts them.
MedImmune anticipates rise in demand
Kathleen Coelingh, senior director for medical affairs at MedImmune, told CIDRAP News that the company is prepared to handle any increased demand for LAIV that would result from the ACIP recommendation. "The good thing is that this was carefully considered over at least 2 years," she said.
She said the company plans to make 18 million doses of FluMist Quadrivalent for the US market for the 2014-15 season, up from the 13 million it produced last season. Coelingh said the company increased the number of doses, anticipating that ACIP might make the recommendation. MedImmune expects to release its first doses in August and can make some late adjustments, as needed, she added.
MedImmune has excess production capacity now, which will gradually increase over the next flu seasons, Coelingh said. She noted that FluMist Quadrivalent already has a sizeable share of the pediatric flu vaccine market and that, even if demand increases and immunization rates go up, the company will be able to respond with enough product.
See also:
Jun 25-26 ACIP agenda
ACIP membership roster
Feb 26 CIDRAP News story "ACIP eyes preference for nasal-spray flu vaccine in kids"