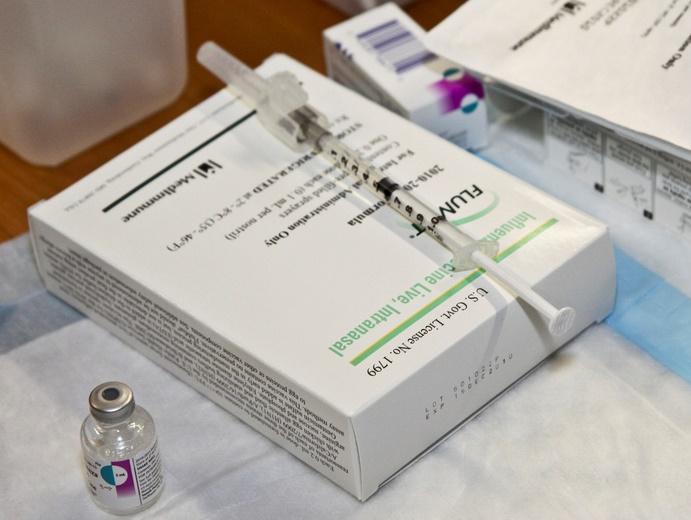
As the season's first reports of influenza deaths appear in the media, some manufacturers and US regions are reporting delays in flu vaccine shipments, but company officials said they are minor and not a cause for concern.
This week local media reported shortages of the pediatric formulation of Sanofi Pasteur's Fluzone vaccine in some areas of Minnesota. And some facilities in the Virginia Peninsula—an area in Virginia that includes Williamsburg, Newport News, and Hampton—reported slow shipments of the FluMist nasal-spray vaccine, made by MedImmune.
A scientist with the St. Paul–based advocacy group Immunization Action Coalition (IAC) said minor glitches are not out of the ordinary and that vaccine distribution is a months-long process.
"Many people forget that flu vaccine is not produced like Dunkin' Donuts where they make it all and then put it all into one box and hand it to you in one batch," said Litjen Tan, PhD, chief strategy officer with IAC.
"It is more like Krispy Kreme, where the doughnuts roll off a conveyer belt over time. Thus, vaccine must arrive over time."
Early-season flu cases, deaths
The vaccine news parallels accounts of early-season flu deaths in North Carolina and Idaho.
Yesterday the Associated Press (AP) reported that the North Carolina Department of Health and Human Services said an elementary school–aged child in the state's Triangle region died last week of flu. He had underlying medical conditions, the agency said.
And an elderly woman in Idaho also died of the flu, KXLY-TV in Spokane–Coeur d'Alene reported. The state's Panhandle Health District said a woman over the age of 65 in Kootenai County died from flu complications on Oct 8. Her case was the first reported in the state this year.
In addition, Maryland reported its first flu case of the season, caused by an H3 strain in an adult, according to the Baltimore Sun, and Kentucky officials confirmed that state's first flu case, the Louisville-based Courier-Journal reported.
Local vaccine delays
Delayed shipments of Fluzone for children 3 years and younger mean that physicians at Children's Hospitals and Clinics of Minnesota have been delaying flu shots for that age-group, the Minneapolis-based Star Tribune reported this week. Sanofi is the only provider of injectable flu vaccine for US kids 3 and younger, but FluMist is an option for children 2 years and older.
Another major Minnesota vaccine provider, MVNA, however, reported a sufficient supply of flu vaccine.
On the Virginia Peninsula, delays in FluMist have been noted throughout the region, though not all providers are affected, the region's Daily Press reported. Craig Parrish, director of pharmacy services for the Virginia Department of Health, said the state received its first shipment of FluMist Quadrivalent—the four-strain version—2 days ago.
Last month, another vaccine maker, GSK, reported that it will fall about 2 million doses short of its earlier estimate of vaccine production because of problems at its Ste. Foy, Que., plant. That situation is also causing delays.
Companies still expect full delivery
Michael Szumera, director of US communications at Sanofi Pasteur, told CIDRAP News, "Sanofi Pasteur has informed customers that shipments of certain presentations of Fluzone influenza vaccine have been delayed. We plan on shipping the majority of doses in October but some of our doses will continue to ship into November."
He added, "Shipments are well under way, and Sanofi Pasteur plans to deliver every dose of the more than 65 million we committed to produce and distribute this influenza season.
"Although the timing of our shipments are later than originally planned, we are doing everything we can to expedite the release and delivery of our Fluzone vaccine shipments. We are working closely with our customers with the common goal of getting patients immunized prior to peak disease season."
Melissa Garcia, MedImmune spokeswoman, said, "We have ample supply of FluMist Quadrivalent for our customers. We are shipping product every week and are on track to deliver between 14 and 15 million doses of FluMist Quadrivalent to our customers nationwide."
When asked specifically about the Virginia situation, Garcia said, "Once our product goes to distributors, they decide how product is allocated and where." She said MedImmune has delivered more than 11 million vaccine doses so far to national distributors, as well as to the Centers for Disease Control and Prevention and the Department of Defense.
The IAC's Tan said that Sanofi's delay was due to problems with production and MedImmune's delay "was at the start of their distribution season, but that is all caught up now." He added that other manufacturers are on pace, "and bioCSL tells us that they are close to complete."
He said that early-arriving flu vaccine in recent years has led to heightened expectations. "Just because we have had a couple of good seasons where vaccine arrived early, that should not be construed to mean that vaccine will always be on hand during August and early September," he said.
News writer Lisa Schnirring contributed to this report.
See also:
Oct 9 AP story
Oct 8 KXLY report
Oct 9 Baltimore Sun story
Oct 9 Courier-Journal article
Oct 8 Star Tribune story
Oct 8 Daily Press story
Sep 4 CIDRAP News item on GSK delays