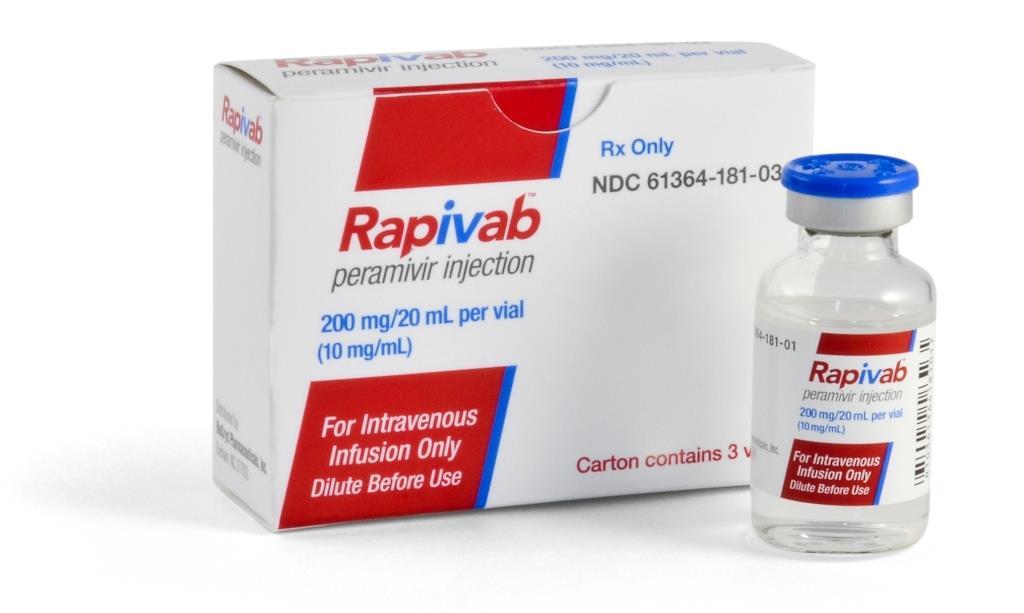
The US Food and Drug Administration (FDA) today announced the approval of the antiviral drug peramivir (Rapivab), making an intravenous (IV) medication available for adult influenza patients who have trouble taking an oral or inhaled antiviral.
Peramivir, made by BioCryst Pharmaceuticals Inc., is a neuraminidase inhibitor (NI), like the licensed drugs oseltamivir (Tamiflu), taken orally, and zanamivir (Relenza), which is inhaled. It is intended to be given in a single dose.
"Rapivab is the third neuraminidase inhibitor approved by the FDA to treat flu infection, but the first approved as an IV formulation," Edward Cox, MD, MPH, of the FDA’s Center for Drug Evaluation and Research, said in an FDA press release. "The availability of a single-dose, intravenous option for the treatment of acute uncomplicated flu allows health care professionals and patients to have a choice based on an individual patient’s needs."
In a BioCryst press release, Richard Whitley, MD, of the University of Alabama at Birmingham, said, "RAPIVAB is the first neuraminidase inhibitor that has shown to be safe and effective as a single-dose, i.v. therapy for patients with acute, uncomplicated influenza, and represents the first new antiviral treatment for influenza approved by the FDA in 15 years."
Faster recovery seen in trial
The FDA said peramivir's efficacy was established in a trial involving 297 patients with confirmed flu who were randomly assigned to receive 300 or 600 milligrams of the drug or a placebo. Overall, patients who received 600 mg of peramivir had symptom relief 21 hours sooner, on average, than those who received the placebo, which is consistent with other drugs in the same class.
Those receiving peramvir 600 mg also recovered from fever about 12 hours sooner than the placebo patients did, the FDA said. Supportive trials confirmed these findings. However, efficacy could not be established in patients with serious flu requiring hospitalization, the agency noted.
Peramivir is intended for certain groups of patients, such as those who have vomiting and diarrhea, for whom a 5-day course of oral capsules would be inappropriate, or those who would have trouble taking an inhaled drug because of upper respiratory symptoms, said Jon Stonehouse, BioCryst's president and CEO.
Other candidates for peramivir would be patients in a hospital emergency department who are at risk for flu complications, a circumstance in which the physician would want to be confident that the patient received the full course of treatment in one dose, Stonehouse said in an interview.
"A lot of times the doctor will prescribe a drug and not know if the prescription will be filled and the patient will get the full course of therapy," he commented.
BioCryst officials noted that peramivir has been licensed in Japan (as Rapiacta) and South Korea (as PeramiFlu) since 2010. The company estimates that more than a million people have been treated with the drug. It was also used in the United States on an emergency basis during the 2009 H1N1 flu pandemic.
The most common adverse reaction to peramvir has been diarrhea, in less than 2% of patients, the company said. Rare side effects have included serious skin reactions, such as Stevens-Johnson syndrome and erythema multiforme.
A big turnaround
The approval of peramivir marks a big turnaround from the situation about 2 years ago, in November 2012, when BioCryst said it would probably give up on seeking a US license for the drug. That followed an interim analysis of a phase 3 trial that showed it had only a small effect in severely ill flu patients.
William P. Sheridan, MB BS, BioCryst's chief medical officer, said peramirivr was developed for both uncomplicated flu and more severe cases resulting in hospitalization. He observed that it's much harder to show efficacy against severe flu illness.
Like other NIs, peramivir is best used within the first 2 days after illness onset, according to the FDA and BioCryst, though studies of other NIs have shown that some benefit is still possible if treatment begins later.
"Patients who come to the hospital with flu and get admitted often have had flu for a long time," Sheridan said. "We were not successful in showing efficacy in hospital studies, but we had efficacy in uncomplicated flu. It's great that we now have the drug approved."
Stonehouse added that peramivir is the first drug discovered by BioCryst to win FDA approval, making the news an important milestone for the company. The firm, based in Durham, N.C., was established in 1986.
Peramirvir was developed with the help of a contract worth $234.8 million from the US Biomedical Advanced Research and Development Authority (BARDA), part of the Department of Health and Human Services.
Stonehouse said peramivir will be available very soon, commenting, "We have trucks actually moving drug to our distributor as we speak."
See also:
Dec 22 FDA press release
Dec 22 BioCryst press release
Related Nov 9, 2012, CIDRAP News story