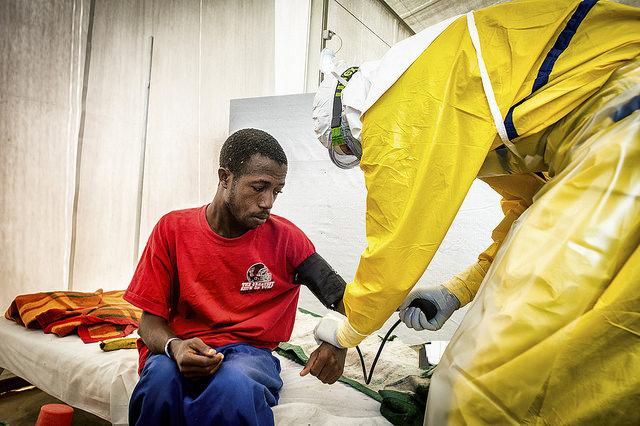
The National Institutes of Allergy and Infectious Diseases (NIAID) announced today that it and Liberia's government have launched the first clinical trial of ZMapp, a monoclonal antibody treatment that has been used on an emergency basis to treat some Ebola patients, especially sick health workers who were evacuated from the outbreak region.
In a statement today the NIAID detailed the design of the trial, which will take place at an Ebola treatment center in Monrovia and in the United States at up to four high-containment treatment facilities, including the National Institutes of Health Clinical Center in Bethesda. Md.
The agency added, however, that steeply dropping Ebola activity in the outbreak region—along with fewer cases likely to be treated in the United States—will likely make it difficult to stick to the original trial design.
"Study investigators anticipate the need for flexibility in the conduct and design of the trial to address the changing nature of the outbreak in West Africa," the NIAID said. "Consideration will also be given to other sites in the outbreak region that express interest."
Clinical trial details
The first phase will be a randomized controlled trial of adults and children to compare ZMapp, made by Mapp Biopharmaceutical, with standard care, which typically includes intravenous fluids, correcting electrolyte imbalances, maintaining oxygen levels and blood pressure, and treating any secondary infections.
Anthony Fauci, MD, NIAID's director, said in the statement that although ZMapp has been used to treat several patients over the past few months, it's difficult to tell if the drug truly worked, because it wasn't administered in a controlled trial setting. "This clinical trial will help us determine if ZMapp and other treatments are safe and effective for use in the current devastating outbreak in West Africa as well as in future outbreaks."
Patients who will get ZMapp will receive standard care, plus three separate intravenous infusions of ZMapp given 3 days apart. The dose will depend on the patient's weight, and those who receive the drug will be monitored for 30 days after hospital discharge and may return to the hospital for follow-up assessment.
Researchers hope to include 100 participants in each arm of the trial, which will first compare ZMapp with standard care to establish a baseline, then evaluate other possible Ebola treatments to gauge which would become the standard of care against which other Ebola interventions will be tested.
NIAID said additional treatments could include TKM-Ebola, favipiravir, convalescent plasma, BCX4430 (an RNA inhibitor developed by BioCryst), and AVI-7537 (an RNA inhibitor made by Sarepta).
The NIAID added that it is collaborating with experts from seven institutions to conduct the trial, which is expected to conclude in December 2016.
Ebola vaccine developments
Meanwhile, groups involved in the development and potential rollout of Ebola vaccines today announced updates.
An official from the World Health Organization (WHO) who spoke to reporters at the close of a 3-day vaccine advisory meeting said an independent group will decide in August at the earliest if an Ebola vaccination campaign should take place in the outbreak region, according to a Reuters report.
Christian Lindmeier, a WHO spokesman, said the recommendation will depend on the results from clinical trials and the course of the epidemic. A trial of the two vaccines that are furthest along in development are under way in Liberia, and other trials are planned for Sierra Leone and Guinea.
Also, researchers involved in human trials of a third Ebola vaccine, a prime-boost product developed by Johnson & Johnson, said the vaccine is being fast-tracked and will be tested soon in African countries that haven't been hit by the outbreak, UK-based Sky News reported today.
The first human trial of the vaccine, conducted by researchers from Oxford Vaccine Group at the University of Oxford, launched in early January. The next phase of the trial is expected to launch soon in Ghana, Tanzania, and Kenya, with large-scale testing planned for later this year, according to the report.
Outbreak numbers, herb-derived treatment candidate
- The number of confirmed, suspected, and probable Ebola cases in the three main outbreak countries is now at 23,825, with the addition of 44 cases since yesterday, the WHO said today. Meanwhile, 23 more deaths were reported, pushing the overall fatality count to 9,660. The totals reflect cases reported through Feb 25 for Guinea and Sierra Leone and through Feb 23 for Liberia.
- Researchers from Texas and their German collaborators have determined that a small molecule called tetrandrine, derived from an Asian herb, can protect mice from experimental Ebola infection, according to a study today in Science. The tetrandrine findings grew out of studies to identify calcium channel signals that were involved in Ebola infection at the cellular level. The team fleshed out the key role of two pore channels that had not been shown for any other virus, which sent them searching for drugs targeting that interaction for use as possible treatments. Some cardiac drugs have the potential to turn the calcium sensor on and off, and in their screening of small molecules, the investigators identified tetrandrine, which they said was most potent, well tolerated, and less cytotoxic, and could be given in a smaller dose. When given to mice, the molecule stopped virus replication and prevented most of the animals from getting infected, which the team notes makes it a candidate for further animal studies.
See also:
Feb 27 NIAID press release
Feb 27 Reuters story
Feb 27 Sky News report
Feb 27 WHO update
Feb 27 Science abstract
Feb 26 Texas Biomedical Research Institute press release on the study