The pace of new Ebola cases in the Democratic Republic of the Congo (DRC) saw no let-up today, with 10 more illnesses reported across five locations in the affected area, the country's health ministry said in its latest update.
Several areas affected
The 10 new cases push the overall outbreak total to 515 cases, 467 of them confirmed and 48 listed as probable.
According to today's update, the new cases are in Kyondo (4), Mabalako (2), Komanda (2), Kalunguta (1), and Beni (1).
Mabalako was one of the outbreak's early hot spots, and health officials have said they are worried about a resurgence of cases in it and other similar areas. The health ministry said today that a case-patient in Mabalako yesterday began having symptoms in his home near Biena, and investigators found that the individual contracted the virus from a patient in Aloya City in Mabalako health zone who died at an Ebola treatment center on Dec 3.
Seven more deaths were reported today, raising the fatality count to 303. The patients who died were from Kyondo, Katwa, Beni, Mabalako, and Komanda. All except the patient in Beni died in the community, a situation known to pose a high risk of disease spread.
Investigations continue into 96 suspected Ebola cases.
Youngest patient discharged
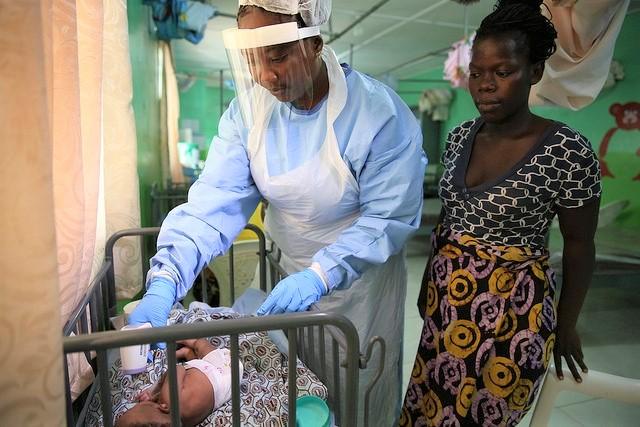
Meanwhile, the ministry said a baby born on Oct 31 to a mother with Ebola who died during childbirth is recovering after contracting the virus. The baby, the youngest known person to be infected in the outbreak, was admitted to a treatment center 6 days after birth and was discharged yesterday.
Doctors and other health workers took turns at the baby's bedside 24 hours a day to monitor treatment, the ministry said, adding that care-team nannies are Ebola survivors who can care for sick children while wearing lighter protective gear.
"They play a fundamental role in caring for sick children who need more attention and a reassuring presence at their side during treatment," the update said.
mAb114 licensing agreement
In other related Ebola developments, Ridgeback Biotherapeutics, a biotechnology company based in Miami, today announced an agreement with the US National Institute of Allergy and Infectious Diseases (NIAID) to license mAb114, an experimental treatment for Ebola.
The drug is a monoclonal antibody that is being used on an emergency, compassionate-use basis to treat Ebola patients in the DCR outbreak. In November, randomized controlled trials of mAb114 and other treatments launched in the country's outbreak region.
The company said in a press release today that mAb114 has already been through a phase 1 safety study, and preclinical studies in nonhuman primates suggested protection against Ebola with one-dose treatment.
In today's announcement, Ridgeback said the patent license agreement with NIAID covers intellectual property related to mAb114. Wendy Holman, the company's chief executive officer and cofounder, said, "We are thrilled to have partnered with NIAID's Vaccine Research Center ('VRC') on mAb114 and will continue the incredible work that the VRC team has done."
She said the company is grateful to the people and groups that have supported mAb114, as well as Ebola treatments and vaccines. "Your efforts have been heroic and will continue to be essential in this fight against a disease which knows no boundaries," she added.
mAb114 was isolated from the blood of a patient who survived an Ebola infection in a 1995 outbreak in the city of Kikwit in the DRC. An NIAID VRC research team led by Nancy Sullivan, PhD, plus collaborators from the DRC, found that the patient still had Ebola antibodies against Ebola 11 years after infection. After isolating and testing the most favorable antibodies in lab and animal studies, they found that mAb114 was the most promising.
See also:
Dec 13 DRC update
Dec 12 World Health Organization Ebola situation report
Dec 13 Ridgeback Therapeutics press release