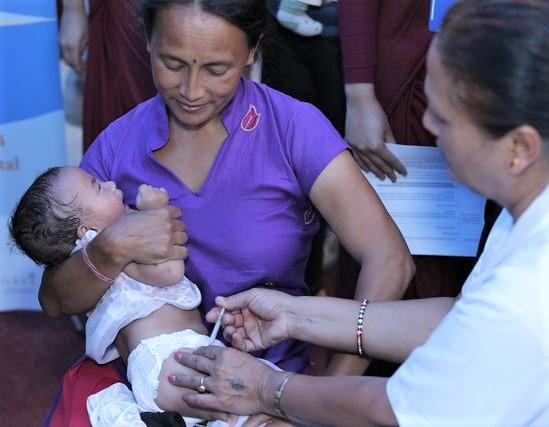
In new polio developments, Pakistan has reported four more wild poliovirus type 1 (WPV1) cases, and research findings lend support to the use of fractional dosing for inactivated poliovirus vaccine (IPV).
New Pakistani cases
Pakistan this year has reported a small but steady stream of new WPV1 cases and has also seen an increase in deadly attacks on polio vaccination teams and security details that protect them.
According to the latest weekly update from the Global Polio Eradication Initiative (GPEI), two of the four new cases are from Bannu district in Khyber Pakhtunkhwa province in patients who had paralysis onsets of Apr 18 and Apr 26. Another is from North Waziristan district in the same province in a patient whose paralysis symptoms began on Apr 22. The fourth case involves a patient from Larkana district in Sindh province whose symptoms began on Apr 17.
Pakistan—one of three countries in which WPV1 is still considered endemic—has now reported 15 cases this year, the most of any country. Also, its number of cases this year has passed the 12 reported for all of 2018.
Positive environmental sample in Iran
Elsewhere, Iran reported WPV1 in environmental sample collected on Apr 22 from Seestan and Balochistan province, located in the southeast of the country on the border with Pakistan and Afghanistan.
Genetic sequencing suggests the virus found in the environmental sample is related to WPV1 circulating in Karachi, Pakistan.
GPEI said the country's health ministry and local officials are conducting a detailed investigation, and an initial risk assessment points to limited public health implications, given Iran's very high levels of routine immunization coverage and disease surveillance. "However, this event further underlines the risk of international spread of WPV1 from Pakistan/Afghanistan," GPEI said.
Role of fractional vaccine dosing
With the global shift oral vaccine to IPV well under way, experts have been eyeing fractional dosing as a way to make immunization more affordable, and the strategy has also been crucial for stretching polio vaccine doses amid IPV supply shortages.
To answer key questions about the protection afforded by fractional dosing, researchers from the US Centers for Disease Control and Prevention (CDC) and their partners in Bangladesh conducted a head-to-head study that compared two fractional intradermal doses in babies ages 6 weeks and 15 weeks old with one full IPV dose given intramuscularly at 14 weeks of age.
Another goal of their study, which was funded by the CDC, was to assess the immunogenicity of a fractional dose for outbreak response purposes. They reported their findings yesterday in The Lancet.
The open-label, randomized, controlled trial took place from Sep 1, 2016, to May 2, 2017, at two clinics in Dhaka, Bangladesh, and enrolled 1,076 participants. Healthy 6-week-old babies were randomly assigned to one of four groups: full-dose IPV at 14 weeks, followed by full-dose booster at 22 weeks; full dose at 14 weeks followed by a fractional-dose booster at 22 weeks; full dose at 6 weeks followed by a fractional dose at 22 weeks, and a fractional dose at 14 weeks followed by a fractional booster at 22 weeks. The full dose was given by intramuscular injection, and the fractional dose was given by needle intradermally.
The researchers examined vaccine response to all three poliovirus types at 22 weeks, meant to assess routine immunization, and at age 26 weeks, meant to evaluate outbreak response.
They found that the two intradermal fractional doses were more immunogenic, with a 16% to 36% higher response compared with one IPV dose and antibody titers that were five to six times as high. Babies who received IPV at 14 weeks of age had a 98% or higher cumulative vaccine response to all serotypes, regardless of whether they received IPV or a fractional-dose booster.
Also, the team found that a fractional-dose booster was noninferior to an IPV booster for all babies who had received IPV when they were 14 weeks old.
They concluded that the study supports World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) recommendations to introduce two fractional doses at 6 and 14 weeks and to use a fractional-dose booster in outbreak response settings to rapidly increase immunity in kids previously vaccinated with IPV or a fractional dose.
Continued IPV shortages
In a related commentary in the same issue, Roland Sutter, MD, and Michel Zaffran, MEng, both from the WHO's polio eradication department, wrote that the new study is an important addition to the growing scientific literature about fractional-dose IPV and answers important questions about the use of fractional doses.
"Unfortunately, the shortages will last longer than anticipated, despite substantial efforts by manufacturers, and the fractional-dose approach might need to be further expanded to indications beyond routine immunization," they wrote.
Though there are still vaccine program challenges that revolve around intradermal vaccine administration, especially in countries with weak health workforces and rigid rules for off-label use, the importance of a fractional-dose strategy can't be overemphasized, Sutter and Zaffran said.
"This approach allowed countries to make best use of scarce IPV supplies and participate in the globally synchronised withdrawal of the Sabin type 2 vaccine," they wrote.
See also:
May 17 GPEI weekly update
May 16 Lancet abstract
May 16 Lancet commentary