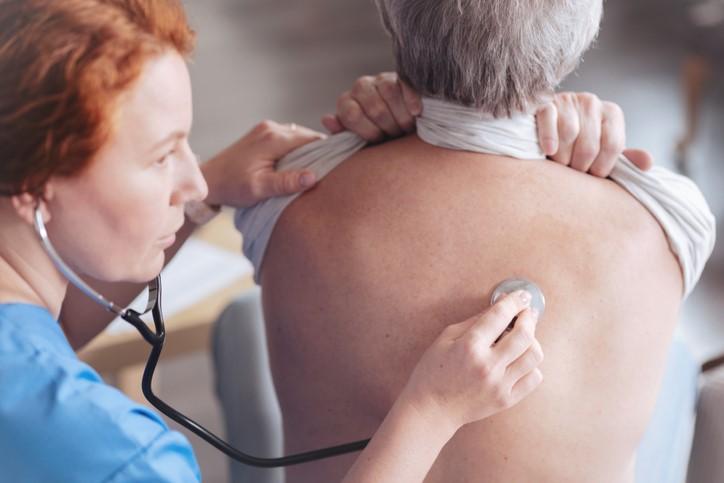
The results of a phase 3 clinical trial published in JAMA demonstrate that oral lefamulin, a novel antibiotic recently approved by the US Food and Drug Administration (FDA), is non-inferior to oral moxifloxacin for the treatment of community-acquired bacterial pneumonia (CABP).
Lefamulin is a first-in-class, semi-synthetic pleuromutilin antibiotic designed to inhibit the synthesis of bacterial protein, which is required for bacterial growth. It's indicated for treatment of CABP caused by the most common gram-positive and gram-negative bacteria associated with the disease, including Streptococcus pneumoniae, methicillin-susceptible Staphylococcus aureus, Mycoplasma pneumoniae, and Haemophilus influenzae.
In the Lefamulin Evaluation Against Pneumonia (LEAP) 2 trial, a team led by researchers from drug sponsor Nabriva Therapeutics of Dublin randomized 738 patients with CABP 1:1 to receive either 5 days of oral lefamulin (370 patients) or 7 days of oral moxifloxacin (368 patients).
The FDA primary end point was early clinical response at 96 hours after the first dose of either study drug in the intention-to-treat (ITT) population. The secondary end points were investigator assessment of clinical response at test of cure in the modified ITT population and in the clinically evaluable population. The non-inferiority margin was 10%.
The early clinical response rates in the ITT population were 90.8% for the lefamulin group vs 90.8% for the moxifloxacin group (difference, 0.1 percentage points). Rates of investigator assessment of clinical response success were 87.5% with lefamulin and 89.1% with moxifloxacin in the modified ITT population (difference, –1.6 percentage points) and 89.7% and 93.6%, respectively, in the clinically evaluable population (difference, –3.9 percentage points) at test of cure.
The overall incidence of treatment-emergent adverse events was 32.6% with lefamulin and 25.0% with moxifloxacin, with gastrointestinal-related adverse events occurring in 17.9% of patients who received lefamulin and 7.6% of patients who received moxifloxacin. Most reported events were either mild or moderate in severity. The most common treatment-emergent adverse events were diarrhea (12.2%), nausea (5.2%), and vomiting (3.3%) in the lefamulin group and nausea (1.9%), headache (1.6%), and urinary tract infection (1.6%) in the moxifloxacin group.
Cost, tolerability are potential issues
CABP is one of the most common infectious causes of illness and hospitalization, especially in the elderly, and a common indication for antibiotic use. According to the Centers for Disease Control and Prevention, roughly 1 million people seek hospital care for CABP each year, and 50,000 people die from the disease. CABP is typically treated with macrolide and fluoroquinolone antibiotics.
In an accompanying editorial, Preeti Malani, MD, of the department of internal medicine at the University of Michigan, writes that lefamulin is "an important addition to the current antibiotic armamentarium." But she also notes that tolerability (especially diarrhea and vomiting) and cost will be issues for the drug. The IV formulation of lefamulin will have a wholesale price of $205 per day, and the oral treatment will cost $275 per day.
"This is several-fold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP," she writes.
The FDA approved oral and intravenous (IV) lefamulin for the treatment of CABP in August based on the data from LEAP 2 and the previously published +LEAP 1 trial, in which 5 to 7 days of IV/oral lefamulin demonstrated non-inferiority to 7 days of IV/oral moxifloxacin with or without linezolid. The drug will be sold under the brand name Xenleta.
See also:
Sep 27 JAMA study
Sep 27 JAMA editorial
Aug 20 CIDRAP News story "FDA approves novel antibiotic for community-acquired pneumonia"