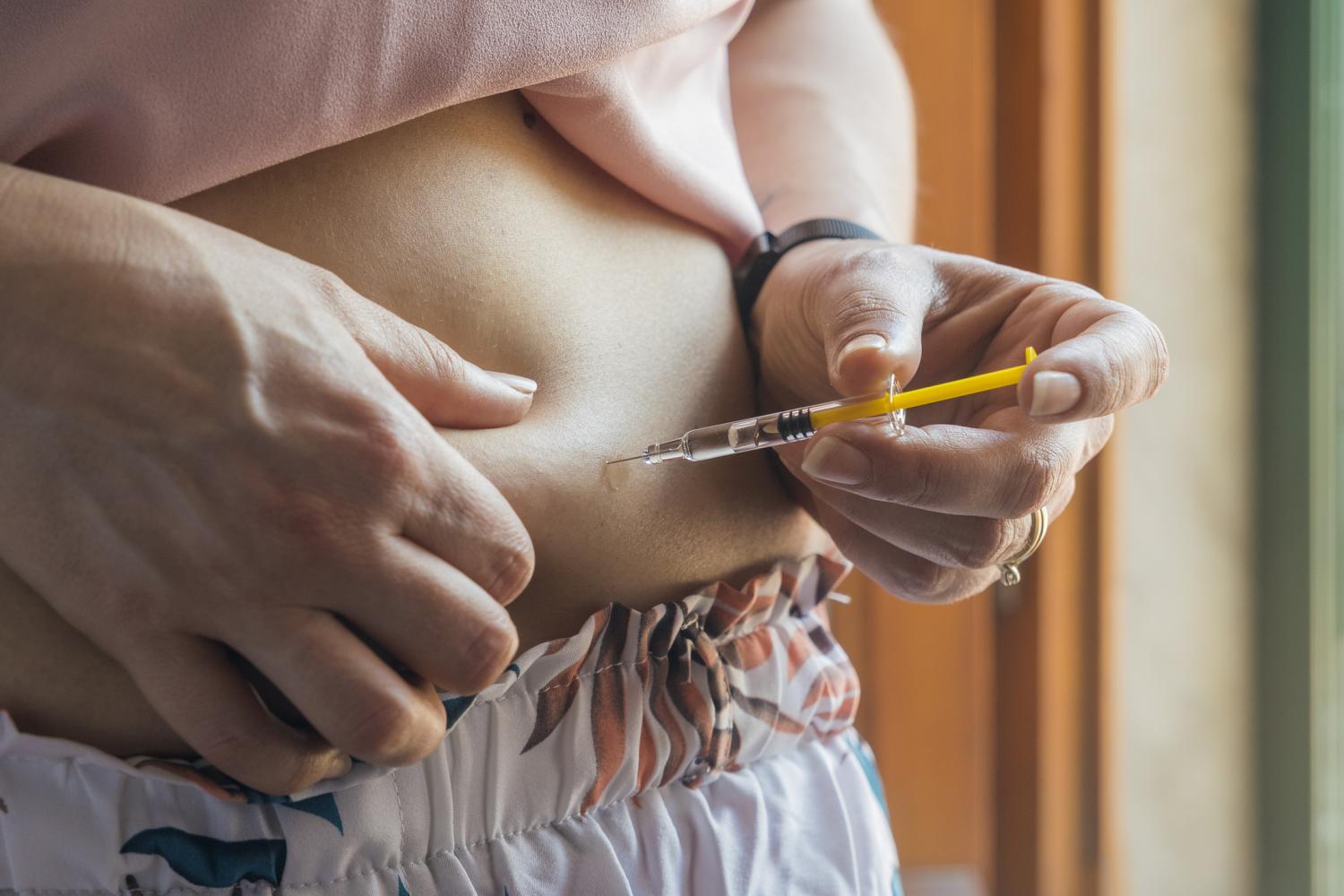
Amid spotty access to semaglutide (brand names, Ozempic and Wegovy), people seeking the injectable drug for weight loss have begun ordering it from alleged doctors on TikTok, online pharmacies, medical spas, and compounding pharmacies peddling nonexistent "generic" versions of the drug.
Ozempic, a diabetes drug also prescribed off-label for weight loss, was in shortage for roughly 6 months after celebrities and social media users began using it to lose weight, leaving some diabetes patients without their medication.
Regulators, pharmacists, and healthcare providers are alarmed by the trend, reported in the New York Times, because Ozempic maker Novo Nordisk doesn't sell the drug for compounding, and there is no Food and Drug Administration (FDA)-approved generic version. Ozempic is currently listed as in shortage by the American Society of Health-System Pharmacists (ASHP), but the FDA no longer lists it as such.
Compounded injectables pose greater risk
"Compounding pharmacies are useful for many patients in need of hard-to-find medications or patient-specific formulations," said David Margraf, PharmD, PhD, pharmaceutical research scientist at the Resilient Drug Supply Project (RDSP). "However, the compounding of semaglutide, particularly by unauthorized sources, is a cause for concern."
RDSP is part of the University of Minnesota's Center for Infectious Disease Research and Policy (CIDRAP), publisher of CIDRAP News.
Compared with FDA-approved drugs, compounded injectable drugs carry a higher likelihood of improper formulation, inaccurate dosing, or contamination. "Consequently, compounded versions of semaglutide have not undergone rigorous evaluation by the FDA for safety, effectiveness, and quality assurance," Margraf said. "Unfortunately, there have been instances where patients have suffered injuries due to poorly compounded drugs in the past."
Compounding is the mixing and altering of a drug to tailor it for use by patients who need a reformulated version. Under federal law, these facilities can compound drugs using only active ingredients originating in FDA-registered sources—and only during FDA-listed drug shortages.
But although state boards of pharmacy license and inspect compounding facilities, the FDA inspects only the ones seen as a safety risk—meaning the sector is largely unregulated.
Boards of pharmacy, drug maker taking action
In late April, the FDA recognized that some compounders were substituting untested and unproven salt forms of semaglutide (ie, semaglutide sodium and semaglutide acetate) rather than its base form. "We are not aware of any basis for compounding a drug using these semaglutide salts that would meet federal law requirements that limit the types of active ingredients that can be used in compounding," the FDA said in a letter to the National Association Boards of Pharmacy.
The North Carolina Board of Pharmacy, West Virginia Board of Pharmacy, and Mississippi Board of Pharmacy issued warnings about possible enforcement consequences of the use of semaglutide salts in compounding.
A Novo Nordisk representative told the New York Times that the firm is taking actions such as sending cease-and-desist letters against "entities that are engaging in the unlawful sale of compounded semaglutide, disseminating false advertising, and infringing its trademarks."