Two studies yesterday in JAMA Otolaryngology - Head & Neck Surgery explore sudden hearing loss after COVID-19 vaccination, one finding no link and the other showing a marginally higher incidence among Pfizer/BioNTech vaccine recipients.
Relative number of reports declined over time
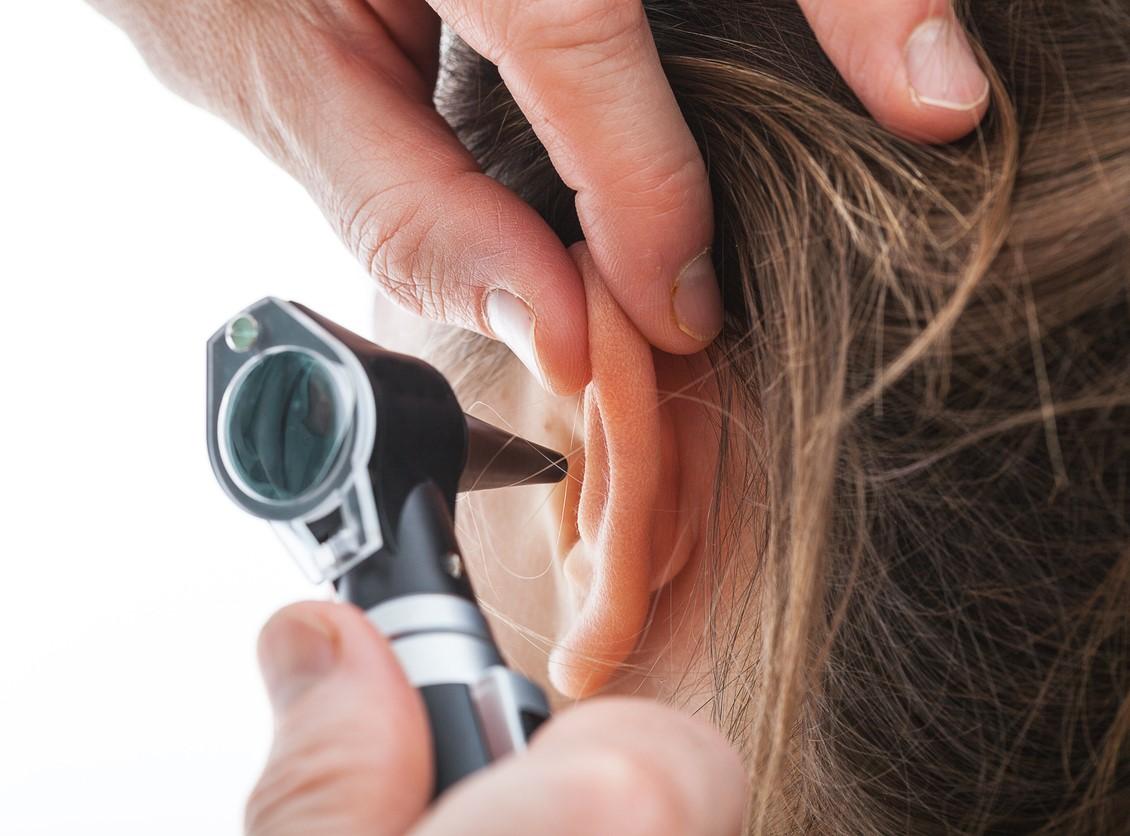
A team led by Johns Hopkins University researchers investigated 555 cases of sudden sensorineural hearing loss (SSNHL) among adults within 3 weeks of COVID-19 vaccination reported to the Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System (VAERS) during the first 7 months of the US COVID vaccine rollout (Dec 14, 2020, to Jul 16, 2021).
The patients had received the Pfizer, Moderna, or Johnson & Johnson (J&J) COVID-19 vaccine. The study period spanned the administration of nearly 187 million vaccine doses in the United States.
In addition to the cross-sectional study, the authors also analyzed a multicenter, retrospective case series of 21 patients at two hospitals and one community practice who experienced SSNHL after COVID-19 vaccination.
SSNHL is unexplained hearing loss occurring all at once or gradually over a few days. The researchers noted that anecdotal reports of post-COVID vaccination have recently emerged among otolaryngologists and the public.
"Otolaryngologists encounter increasing challenges to promoting public health conduct recommended during the pandemic when they are counseling and evaluating patients who have developed SSNHL and reported a temporal association with COVID-19 vaccination," the study authors wrote.
Average age of the 555 patients was 54 years, 55% were women, and average time from vaccination to SSNHL onset was 6 days. The yearly incidence of SSNHL was 0.6 to 28.0 cases per 100,000 people. Of all incidents, 55.0% occurred after the Pfizer vaccine, 40.0% involved Moderna, and 5.0% involved J&J.
Incidence was comparable with all three vaccines, with 0.16 cases per 100,000 doses for Pfizer and Moderna, and 0.22 per 100,000 for J&J. One patient developed SSNHL 14 days after receiving the first Pfizer dose and improved after treatment with oral and intra-ear steroids only to worsen again after the second dose.
The number of SSNHL reports peaked in the last week of March 2021, which corresponded to the most vaccine doses (16,177,521) administered over 1 week since the vaccine rollout began. But each week, the relative number of SSNHL reports declined when accounting for the number of doses administered across the country, from 1.10 reports per 100,000 doses in December 2020 to 0.01 reports per 100,000 in June 2021.
Average age of the 21 case-series patients was 61 years, 61.9% were women, and average time to SSNHL onset was 6 days. Six patients (28.6%) had a preexisting autoimmune disease. Among the 14 patients with posttreatment hearing data, 8 (57.1%) improved after treatment.
The researchers said they could not identify any population-level link between COVID-19 vaccination and SSNHL but could not rule out individual-level associations, as in the patient who had hearing loss after each dose.
"Further prospective investigation is needed to identify any potential associations between COVID-19 vaccination and SSNHL in some individuals," they wrote. "It is important that clinicians report all suspected COVID-19 vaccine–associated AEs [adverse events] rigorously and accurately to VAERS to allow verification and future performance of systematic vaccine safety studies."
Rare occurrence, good prognosis
In a second study, this one from Israel, researchers examined SSNHL cases among 2,602,557 Pfizer COVID-19 vaccine recipients at Clalit Health Services from Dec 20, 2020, to May 31, 2021. They compared SSNHL cases occurring within 21 days after the first and second vaccine doses with the expected number of cases based on data from 2018 and 2019.
Participants included Israelis 16 years and older who received the first vaccine dose from Dec 20, 2020, to Apr 30, 2021, and the second dose from Jan 10, 2021, to Apr 30, 2021.
Average patient age was 46.8 years, and 51.5% were female. Among the 2,602,557 patients who received the first dose of the Pfizer COVID-19 vaccine, 91 cases of SSNHL were reported, for a 21-day cumulative incidence of 3.50 SSNHL cases per 100,000 people. Of those patients, 93.8% also received the second dose, with 79 reports of SSNHL, for a cumulative incidence of 3.24 per 100,000.
The incidence of SSNHL was 60.77 (95% confidence interval [CI], 48.29 to 73.26) per 100,000 person-years after the first vaccine dose and 56.24 (95% CI, 43.83 to 68.64) per 100,000 after the second. The corresponding incidence rates of SSNHL in 2018 was 41.50 (95% CI, 37.98 to 45.01) per 100 000 person-years, while it was 44.46 (95% CI, 40.85 to 48.07) per 100,000 in 2019.
Age- and sex-weighted standard incidence ratios (SIRs) were 1.35 (95% CI, 1.09 to 1.65) after the first vaccine dose and 1.23 (95% CI, 0.98 to 1.53) after the second. The estimated SIRs after the first dose were more pronounced in females 16 to 44 years old (1.92; 95% CI, 0.98 to 3.43) and 65 years or older (1.68; 95% CI, 1.15 to 2.37). The attributable risks were mostly small, with the highest at 3.74 per 100,000 vaccinees in women 65 years and up.
The highest estimated SIR after the second dose occurred in males aged 16 to 44 years (2.45; 95% CI, 1.36 to 4.07). The attributable risks were mostly small, and the findings were comparable when 2019 was used as a reference to estimate the expected number of SSNHL cases.
While the findings suggest a link between the Pfizer COVID-19 vaccine and SSNHL, the effect size is very small, the authors said. "Considering the small effect size of this association and the good prognosis for patients with SSNHL, the potential influence of this condition on public health appears to be relatively minor," the researchers concluded.
"Considering these findings along with the good prognosis for patients with SSNHL, we suggest that the benefits of the BNT162b2 mRNA [Pfizer] COVID-19 vaccine outweigh its potential association with SSNHL," the researchers wrote. "However, sharing these findings with health care professionals who are involved in SSNHL assessment might lead to early recognition and treatment, which are crucial to improve outcome."
Benefits of vaccination outweigh risks
In a related commentary, Angela Ulrich, PhD, MPH, and Michael Osterholm, PhD, MPH, both of the Center for Infectious Disease Research and Policy at the University of Minnesota, publisher of CIDRAP News, and Maria Sundaram, PhD, MSPH, of Marshfield Clinic Research Institute in Wisconsin, said that the results suggest that further research on the potential association between COVID-19 vaccination and SSNHL may be warranted.
"Neither study identified clear demographic or clinical risk factors associated with sudden sensorineural hearing loss, nor did they address important measurements regarding the severity and duration of hearing loss or the clinical outcomes after treatment," they wrote.
They said, however, that the study data must be regarded in light of the known risk of SARS-CoV-2 infection and the one third of patients who develop long COVID, including chest pain, shortness of breath, muscle pain, anxiety and depression, fatigue, and cognitive symptoms that can last longer than 3 months after diagnosis. SSNHL, on the other hand, can be reversed or reduced if diagnosed and treated early, they noted.
"Although the development of new variants of concern (ie, delta and omicron) have challenged the ability of the vaccines to prevent infection, existing vaccines appear to maintain substantial protection against severe illness," Ulrich and colleagues said. "Most important, COVID-19 vaccines have been found to be beneficial at preventing severe COVID-19 illness, including hospitalizations and deaths."