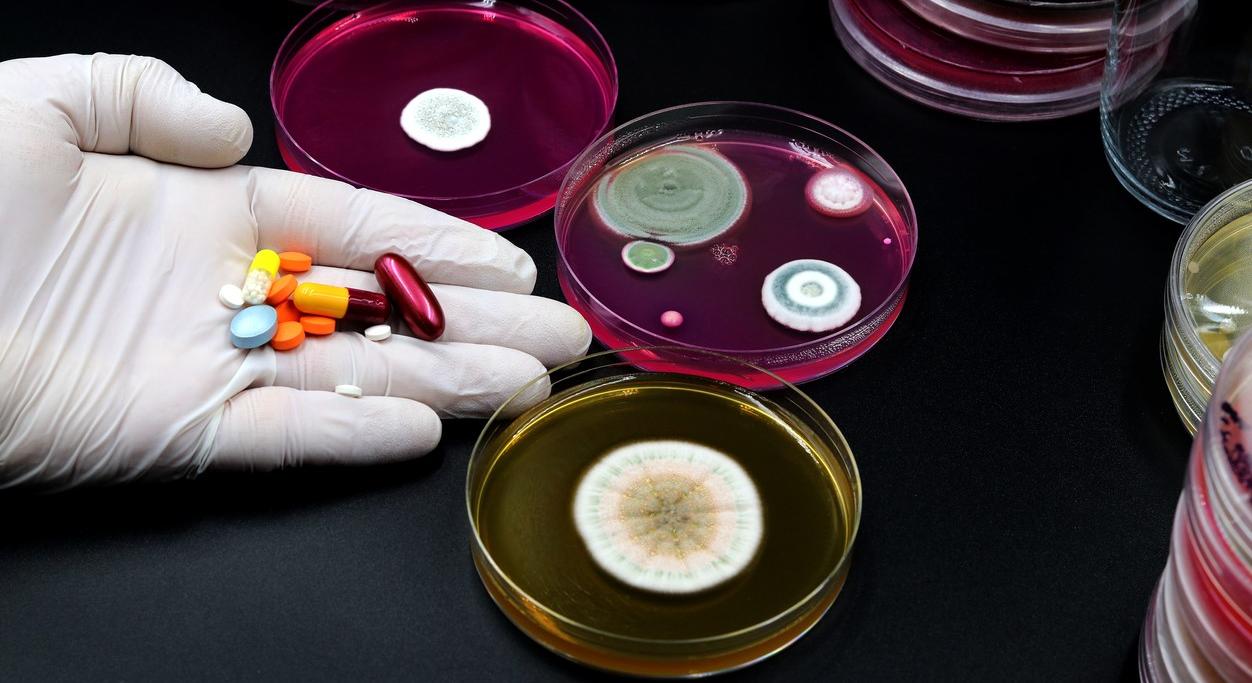
A panel of experts in infectious diseases, antimicrobial resistance (AMR), and drug development today urged US lawmakers to support legislation that could help revitalize the antibiotic development pipeline.
At a hearing held by a subcommittee of the US Senate Committee on Health, Education, Labor & Pensions, the experts spoke about the rising threat AMR poses to public health and modern medicine and the role that infection prevention, antibiotic stewardship, diagnostics, and a bolstered infectious disease workforce can play in addressing the problem.
But they reiterated that Congress and the federal government ultimately have the power to address the biggest problem facing efforts to tackle drug-resistant superbugs—the broken marketplace for new antibiotics.
"The most important thing this subcommittee can do is to advance a policy to establish a pull incentive, such as a subscription model, to spur the discovery and development of novel antimicrobials," said Helen Boucher, MD, dean and professor of medicine at Tufts University School of Medicine.
Support for PASTEUR Act
Joining Boucher on the panel was Christine Ann Miller, president and CEO of drugmaker Melinta Therapeutics, a company that has several antibiotics and antifungals in its portfolio.
Miller said the issue that has prompted many companies to abandon antibiotic research and development (R&D) is neither lack of innovation nor a slow approval process. There are innovative products in the pipeline, she noted, and Congress has worked with the Food and Drug Administration (FDA) to streamline the approval process for new antibiotics that can treat resistant infections.
The problem, Miller explained, is that many of the small companies that develop antibiotics run out of money because newly approved antibiotics are infrequently used. She called for reforms to the reimbursement system and novel mechanisms to "decouple" payment for antibiotics from sales volume.
"The only way to combat these life-threatening infections is to continually innovate newer, safer antimicrobials and ensure that patients have access to these innovations," Miller told the panel. "Unless we see changes to the post-approval side of the equation, the ability to bring these products to patients remains in jeopardy."
To rectify the issue, Miller and Boucher both voiced their support for the PASTEUR (Pioneering Antimicrobial Subscriptions to End Upsurging Resistance) Act, a bill re-introduced by a bipartisan group of lawmakers on April 27. The legislation, which was previously introduced in 2020 and 2021 but has never received a vote despite widespread support, would create a subscription-style payment model for new antibiotics.
The only way to combat these life-threatening infections is to continually innovate newer, safer antimicrobials and ensure that patients have access to these innovations.
Under the model proposed by the bill, the federal government would pay companies up front for access to newly approved, critically needed new antibiotics for drug-resistant infections. In return, the antibiotics would be free for patients covered by federal health programs, and companies would have to ensure they were used appropriately. The hope is that a guaranteed return-on-investment would spur more investment in antibiotic development.
"A subscription model would pay for these antimicrobials based on their value instead of volume to drive private investment in antimicrobial R&D," Boucher said.
One Health and the patient experience
Also on the panel was Michael Apley, DVM, PhD, a professor at the College of Veterinary Medicine at Kansas State University. Apley spoke to the need for a One Health approach to AMR, noting that several of the resistant bacterial pathogens considered to be threats to human health—Pseudomonas, Staphylococcus aureus, and non-typhoidal Salmonella—are also a concern for veterinarians.
"We share a lot of organisms that we have challenges with," he said. "We also share a lot of the same needs for diagnostics and for getting the best information to our practitioners."
Representing the patient experience was Melanie Lawrence, a healthcare advocate living with cystic fibrosis, a genetic lung condition that makes people prone to bacterial infections. Lawrence said that she's been using antibiotics all her life to treat chronic lung infections caused by Pseudomonas and methicillin-resistant S aureus, but the bacteria in her lungs are now resistant to most treatment options. The only one that still works, tobramycin, has become too toxic.
"Without the security of effective antibiotics to make me heal, I find myself living with chronic fear and anxiety about when the bacteria residing in my lungs will act up, or when another infection will take me away from truly living," Lawrence said.
Time is ticking, and we need your help.
Lawrence called on lawmakers to work together to come up with innovative solutions for patients like her.
"Time is ticking, and we need your help," she said.