Biotechnology company Scynexis, Inc., is reporting early but promising results from a phase 3 trial of a novel drug for treating invasive Candida auris infections.
The company will present the results from the first two case studies in the CARES trial at the upcoming European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) in Amsterdam. The single-arm trial is evaluating the efficacy and safety of oral ibrexafungerp in patients with candidiasis caused by C auris, a multidrug-resistant fungus that has triggered deadly outbreaks in healthcare facilities around the world, with mortality rates as high as 60%.
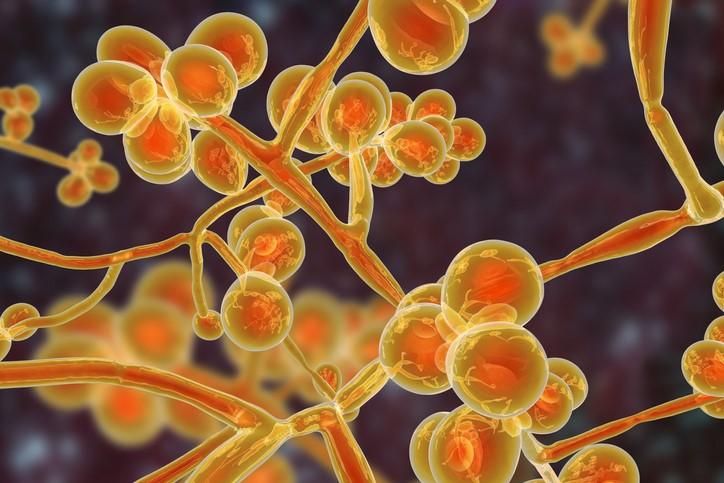
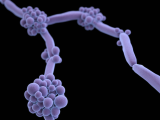
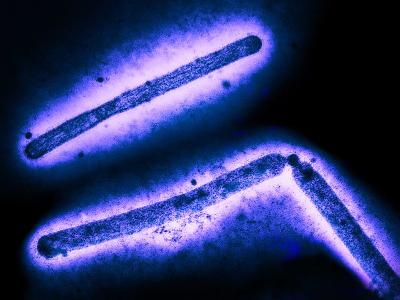
Since it was first identified in 2009 in a patient in Japan, C auris has spread to hospitals in more than 20 countries, proving difficult to eradicate and treat. To date, there are 617 confirmed and probable cases in the United States. The fungus, which mainly affects patients with comorbidities or those who are severely immunocompromised, has shown resistance to the three major classes of antifungals used to treat Candida infections and the ability to persist on hospital surfaces.
Although the trial is in its early stages, Scynexis president and chief executive officer Marco Taglietti, MD, believes the successful treatment of the two patients, and the potent in vitro activity that ibrexafungerp has shown against clinical C auris samples, provides hope that a new treatment option may be on the horizon.
"We think we can beat Candida auris," Taglietti told CIDRAP News. "This is a difficult, frightening pathogen, but we have something to fight it."
Successful treatment in two patients
The first two cases from the CARES trial, which is enrolling patients in the United States and India, involved a 54-year-old man and a 64-year-old woman with C auris bloodstream infections and multiple comorbidities at a hospital in New Delhi. The man had diabetes, acute ischemic stroke, a brain abscess, and pneumonia, and the woman had diabetes, chronic kidney disease, and lower respiratory infection. Both patients were in septic shock.
The patients were initially treated with other antifungals (fluconazole and micafungin), but the C auris infection remained and the investigators initiated treatment with ibrexafungerp. The drug cleared the infections from both patients' blood and was well tolerated. The man completed 17 days of treatment and did not experience a recurrence over 6 weeks, but ultimately died of a different bacterial infection. The woman completed 22 days of treatment and remains free of C auris.
"These two cases are really very positive," Taglietti said.
Taglietti noted that C auris is a major problem in India, with a prevalence of 3% to 5% in some Indian hospitals. He said finding a treatment for the fungus is a priority because while C auris infections have been limited to hospitals, there may be immunocompetent patients who are colonized with it but show no symptoms. If they aren't identified through screening, these patients could spread C auris outside of hospitals.
"If Candida auris starts to spread around, we may start to have more problems," he said.
Taglietti added that while the evidence is from only two patients, the results build on the evidence that's been established on the drug's efficacy against C auris. A 2017 study by Scynexis and researchers with the US Centers for Disease Control and Prevention found that ibrexafungerp showed potent activity against a panel of 100 C auris isolates from around the world, and two animal studies showed the drug was effective in treating invasive C auris infections.
"The full weight of evidence suggests this drug can have an important role against Candida auris," he said.
CARES trial investigators are estimating an enrollment of about 30 patients, with an estimated completion date of May 15, 2021.
Scynexis will also be presenting positive results from the FURI study—a phase 3 trial evaluating ibrexafungerp in the treatment of patients with Candida infections that are intolerant or refractory to standard-of-care treatment—at ECCMID. Among them are two cases of successful treatment of patients with Candida spondylodiscitis, a rare and difficult-to-treat infection of the vertebral disc space and vertebral bone.
"These two cases show how an oral antifungal can be helpful not only for Candida auris…but can also be helpful for other difficult-to-treat fungal infections," Taglietti said.
Raising awareness of fungal infections
Ibrexafungerp is the first candidate from a novel class of structurally distinct glucan synthase inhibitors called triterpenoids. The Food and Drug Administration has granted the drug qualified infectious disease product (QIDP) and Fast Track designations for the treatment of invasive candidiasis, invasive aspergillosis, and vulvovaginal candidiasis. These designations are intended to incentivize and expedite the development of drugs for difficult-to-treat infections.
Taglietti said he hopes the recent media interest in C auris infections will raise the profile of antifungal resistance and the need for new antifungal drugs, issues that haven't received as much attention from policymakers as have antibiotic resistance and antibiotic development.
"I hope this will increase the awareness of fungal infections, and the fact that they are as dangerous and difficult to treat as bacterial infections, and sometimes even worse," he said. "More support for development of antifungals is critical."
See also:
Apr 13 ECCMID CARES trial abstract
Apr 13 ECCMID FURI trial abstract