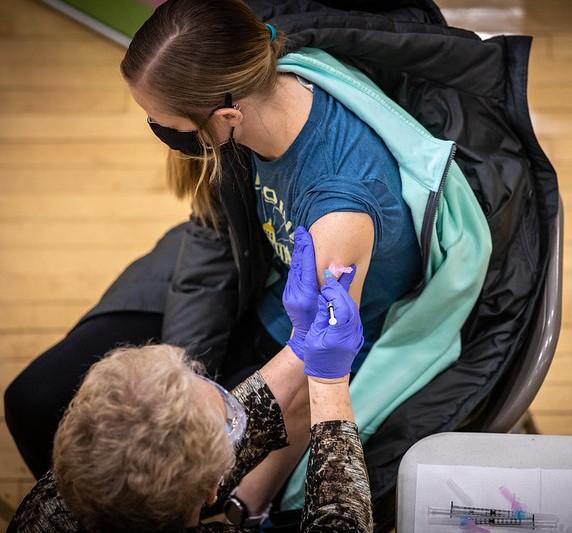
Two doses of the AstraZeneca-Oxford University COVID-19 vaccine were ineffective against mild-to-moderate infections with the B1351 variant first identified in South Africa, according to a phase 1b-2 clinical trial published today in the New England Journal of Medicine.
The double-blind multicenter study, led by scientists at the South African Medical Research Council Vaccines and Infectious Diseases Analytics Research Unit, studied the safety and the efficacy of the AstraZeneca ChAdOx1 nCoV-19 vaccine in HIV-negative adults aged 18 to 64 who received either two standard doses of the vaccine or a placebo in a 1:1 ratio 21 to 35 days apart from Jun 24 to Nov 9, 2020. Median follow-up after the second dose was 121 days.
10.4% effectiveness against variant
Of the 750 participants vaccine recipients, 19 (2.5%) developed mild to moderate COVID-19 more than 14 days after the second dose, compared with 23 of 717 placebo recipients (3.2%). The incidence of COVID-19 among the vaccine group was 73.1 per 1,000 person-years, compared with 93.6 per 1,000 person-years among the placebo group, for an efficacy of 21.9% (95% confidence interval [CI], -49.9 to 59.8).
Of the 42 total cases of COVID-19, 39 (92.9%) were caused by B1351, for a vaccine effectiveness against this variant of 10.4% (95% CI, -76.8 to 54.8). All 42 cases were mild to moderate, and no patients were hospitalized.
"In this trial, we found that two doses of the ChAdOx1 nCoV-19 vaccine had no efficacy against the B.1.351 variant in preventing mild-to-moderate COVID-19," the authors wrote. "The lack of efficacy against the B.1.351 variant should be considered in the context of the 75% efficacy (95% CI, 8.7 to 95.5) in preventing mild-to-moderate COVID-19 with onset at least 14 days after even a single dose of ChAdOx1 nCoV-19 vaccine that was observed before the B.1.351 variant emerged in South Africa."
Second-generation vaccines in development
Comparison of pseudovirus and live-virus neutralization assays against the original D614G strain and B1351 used to test serum from 25 participants 14 days after the booster dose suggested that both were more resistant to B1351 in samples from vaccinees than in samples from placebo recipients.
Serious adverse event rates were similar between the vaccine and placebo groups. Only one severe vaccine-related event occurred, a fever of 40°C (104°F) following the first dose; the fever cleared within 24 hours, and no adverse events were seen after the participant's second dose.
The authors cautioned that the lack of severe COVID-19 cases in the study was likely a reflection of the relatively young mean age of participants (30 years) and thus that the trial could not determine whether the AstraZeneca vaccine is effective against severe infection with the B1351 variant.
They said that the degree to which the efficacy of other COVID-19 vaccines could also be affected by variants with mutations comparable to those of B1351 and P1, the strain first identified in Brazil, may depend on the extent of neutralizing antibody generated by vaccination.
"Whether an enhanced antibody response resulting from a longer interval between the first and second doses of the ChAdOx1 nCoV-19 vaccine, as described elsewhere, might confer better residual neutralizing antibody activity against the B.1.351 variant than that observed in our trial is not known," the researchers wrote.
They concluded by saying that although development of second-generation COVID-19 vaccines against strains such as B1351 and P1 has begun, the only vaccines likely to be available for the rest of 2021 are formulated against the original virus. Until then, the AstraZeneca vaccine is probably going to be the most accessible and cheapest coronavirus vaccine.
"Deliberations on the utility of the ChAdOx1 nCoV-19 vaccine also need to be made in the context of ongoing global spread and community transmission of the B.1.351 variant and the evolution of other SARS-CoV-2 lineages that include similar mutations," the authors said.
In early February, South African health officials paused the rollout of AstraZeneca-Oxford vaccine to investigate reports that it offered little protection against mild-to-moderate disease. It switched to using the Johnson & Johnson vaccine to immunize healthcare workers.