In the absence of a federal testing strategy, surging COVID-19 cases, and the looming 2020-21 school year, the governors of Maryland, Louisiana, Massachusetts, Michigan, North Carolina, Ohio, and Virginia—three Republicans and four Democrats—announced they joined a new interstate compact to receive more than 3 million point-of-care COVID-19 antigen tests.
The compact was negotiated via Larry Hogan, governor of Maryland and chair of the National Governors Association, and the Rockefeller Foundation. According to Hogan's office, as of today the states are in discussions with Becton Dickinson and Quidel, US manufacturers of antigen tests authorized by the Food and Drug Administration, to buy 500,000 tests per state.
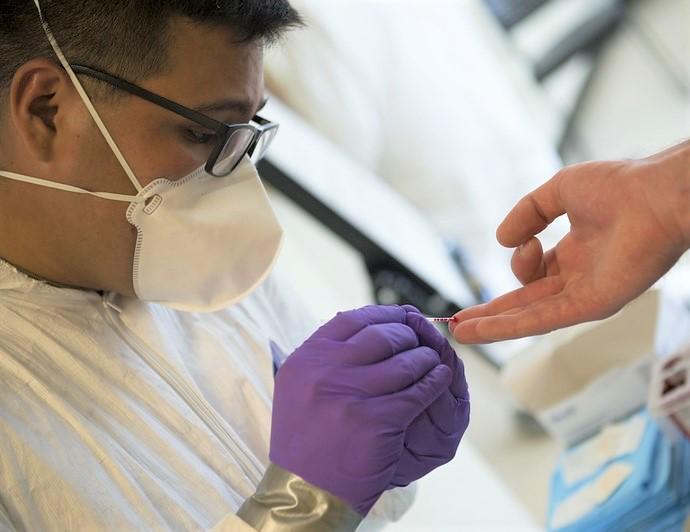
Rapid antigen tests deliver results within 30 minutes and can be used to quickly assess the scope of an outbreak in congregate settings, including nursing homes and schools. They are especially helpful—if readily available—in identifying asymptomatic people who may not seek out testing on their own.
"With severe shortages and delays in testing and the federal administration attempting to cut funding for testing, the states are banding together to acquire millions of faster tests to help save lives and slow the spread of COVID-19," said Hogan in a statement.
Ashish Jha, MD, MPH, director of the Harvard Global Health Institute, praised the efforts on Twitter, and said antigen testing could help solve the testing crisis that has plagued the United States since February. More recently, states have complained about a lag time in testing results and a shortage in chemicals used to process the tests.
If people have to wait more than 24 to 72 hours for test results, efforts to contact trace and quickly quarantine can be lost. According to recent survey in the New York Times, the median turnaround time in most states is 3 days, with minorities reporting wait times of 4 to 5 days.
Chicago plans on distance learning; Mississippi struggles
Chicago Public Schools announced today that the 2020-21 school year will begin online and continue with full distance learning at least through Nov 6. Chicago is the nation's third-largest school district. The largest, Los Angeles and New York City, have decided to proceed with distance learning and a hybrid model, respectively.
Chicago Public Schools said its decision was largely shaped by parent survey responses, which showed 40% of families of elementary schools students did not plan on sending kids back to the classroom, and more than 30% were undecided.
President Donald Trump maintained his commitment to opening schools on Twitter , but even Republican governors who have supported Trump's school message are struggling to find a way to safely open amid increasing outbreaks.
Tate Reeves, governor of Mississippi, implemented a temporary statewide mask mandate yesterday, and said he was delaying the opening of in-person school in some counties. According to National Public Radio, Mississippi is on track to become the No. 1 state for new coronavirus infections per capita.
The mandate will require mask wearing in all school buildings. The delay in school openings will be in effect only for middle and high schools, with younger learners allowed to return to classrooms on Aug 17.
Today the Infectious Diseases Society of America and its HIV Medicine Association sent a letter to Vice President Mike Pence calling for a nationwide mask requirement.
"While state and local requirements are particularly effective at increasing the use of masks, this is the time for national solidarity as COVID-19 has made significant inroads into rural areas that were initially considered 'safe,' " the letter says.
The letter comes as the United States is approaching 5 million COVID-19 cases. According to the Johns Hopkins University COVID-19 tracker, officials have reported 4,802,275 cases and 157,551 deaths.
Vaccine makers try to enlist minority patients
Today the Wall Street Journal highlighted an important challenge in COVID-19 vaccine trials: recruiting enough black and Hispanic participants. Though the novel coronavirus has hit minority communities the hardest, black and Hispanics are the least likely to participate in trials.
The newspaper said researchers are targeting community leaders, churches and advocacy organizations to educate about the benefits of vaccination.
In other vaccine news, Novavax announced promising phase 1 findings for its recombinant adjuvanted COVID vaccine yesterday. The vaccine was well-tolerated in healthy adult volunteers, and there were no severe adverse events.
And today Johnson & Johnson announced an agreement with the US government as part of Operation Warp Speed, which secures 100 million doses of the company's investigational COVID-19 vaccine.