Rates of worrisome highly drug-resistant Pseudomonas aeruginosa infections in US children have risen sharply over the past decade, according to a study today in the Journal of the Pediatric Infectious Diseases Society.
P aeruginosa is a virulent and opportunistic pathogen that can survive in harsh environments and has evolved several mechanisms to combat antibiotics. These mechanisms enable the bacterium to rapidly develop resistance during the course of treating an infection. Infections caused by P aeruginosa, especially in those who are critically ill or immunocompromised, are associated with longer hospital stays and significant increases in morbidity and mortality.
Carbapenem resistance doubles
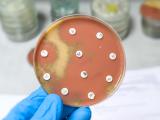
The study, which was based on a susceptibility analysis of more than 77,000 P aeruginosa clinical isolates collected from patients 1 to 17 years old, found that the crude proportion of isolates that were resistant to at least three classes of antibiotics rose from 15.4% in 1999 to 26% in 2012. The proportion of bugs that were resistant to carbapenems, a last-resort antibiotic for pediatric infections, rose from 9.4% to 20%.
In addition, resistance to other antibiotic classes used to treat P aeruginosa—including aminoglycosides, fluoroquinolones, and cephalosporins—also increased.
The analysis further showed that drug-resistant Pseudomonas infections were most likely to be found in children 13 to 17 years old in the Upper Midwest who had been admitted to an intensive care unit with a respiratory tract infection.
"I was very surprised to see that it was such a significant increase," said study author Latania Logan, MD, a pediatric infectious disease specialist at Rush University Medical Center in Chicago. Logan said the proportion of multidrug- and carbapenem-resistant isolates grew by 4% each year of the study. She also noted that the study findings indicate that drug-resistant P aeruginosa, which the Centers for Disease Control and Prevention (CDC) considers a serious threat, may be much more prevalent than previous estimates.
A hardy, resistance-prone bug
P aeruginosa, first identified in 19th century, was initially known for the distinctive blue-green coloration it left on surgical wound dressings. The bug is hardy, needs little nutrition to survive, and "loves harsh environments," Logan said. These attributes enable it to persist in both community and healthcare settings, where it can cause a variety of infections, from minor skin and ear infections to more serious blood, urine, and respiratory infections.
But the bacterium has also developed an arsenal of weapons against antibiotics that have helped make it prone to resistance. These weapons include beta-lactamase enzymes, which break through the chemical composition of antibiotic cells and render them ineffective; efflux pumps that push antibiotics and other toxins out of cells; and proteins that facilitate the formation of protective biofilms. P aeruginosa can also acquire mobile pieces of DNA known as plasmids that can harbor resistance genes.
With all these weapons, it's not surprising that Pseudomonas infections are becoming increasingly drug-resistant. "We knew Pseudomonas is notorious for this," Logan said, which is why she and her colleagues expected to see some increase in resistance, especially in children who are critically ill and receiving antibiotics. "But it's striking how correct we were."
And that's an indication, Logan said, that the use of broader-spectrum antibiotics—such as third- and fourth-generation cephalosporins and fluoroquinolones—could be playing a role in fueling resistance. Because P aeruginosa has developed resistance to so many classes of antibiotic, clinicians have had to expand their antibiotic repertoire, and that in turn is increasing the selection pressure for multidrug- and carbapenem-resistant strains of the bacterium.
"It does seem like it might be reflective of some of our prescribing practices changing over the course of this past decade," Logan said.
Logan noted that she's particularly concerned about rising resistance to carbapenems, which she calls the "last line of defense" for pediatricians. "If we don't have carbapenems, and [Pseudomonas] is resistant to other drugs, then we're going to end up having to use more toxic medications," for the treatment of children.
Public health officials are taking note.
"These are really important data," said Ruth Lynfield, Minnesota state epidemiologist and medical director at the Minnesota Department of Health, noting the large size and nationwide scope of the study. "It's very concerning that we're seeing these increases, and that we have such a high proportion of multidrug-resistant Pseudomonas."
Stewardship could reduce selection pressure
Logan and her colleagues say the findings highlight the need not only for more stringent infection control in pediatric settings but also for antimicrobial stewardship programs. "If you can improve prescribing practices and restrict use of the broad-spectrum antimicrobial agents, you'll reduce that selection pressure issue," Logan explained, because the bacteria won't have to use their resistance mechanisms, which requires energy. "The bacteria don't want to turn on these things if they don't have to."
Stewardship, the study authors add, would be helped by the widespread adoption of rapid molecular diagnostic tests in healthcare facilities, which could guide more accurate antibiotic treatment by quickly indicating to physicians which antibiotics an infection has developed resistance against.
They also say the study indicates the need for more bacterial surveillance, a point echoed by Lynfield. "This is something I think we need to track and monitor, in order to target where we should put in stronger approaches to antibiotic stewardship and infection control," she said.
According to the CDC, an estimated 51,000 healthcare-associated P aeruginosa infections, and roughly 400 deaths, occur each year in the United States. More than 6,000 (13%) of these infections are drug resistant.
See also:
Nov 17 J Pediatric Infect Dis Soc abstract