Apr 26, 2013 (CIDRAP News) – The US Centers for Disease Control and Prevention (CDC) today issued an update on routine preparedness steps it is taking to protect the country if the novel H7N9 virus in China starts spreading among humans.
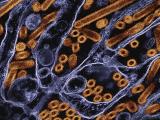
Much of the preparedness work is occurring in labs with H7N9 viruses grown from a sample that the CDC received from China on Apr 11. The CDC said the virus sample replicated well in eggs, which wasn't surprising for an avian influenza virus.
On Apr 15 the CDC started distributing viral samples grown in the eggs to five labs, including one at the US Department of Agriculture. Facilities working on the virus must have proper permits and conduct work in enhanced biosafety level 3 (BSL-3) containment.
Experiments on the virus have already yielded some findings. A study on how the virus spreads has shown that it spreads between animals through close contact, which was expected, the CDC said. Another important question is if the H7N9 virus can be spread by respiratory droplets, and the CDC said those studies are ongoing.
The CDC's lab experts have also used the virus to assess if it is susceptible to oseltamivir (Tamiflu) and zanamivir (Relenza), two neuraminidase inhibitors that are recommended for treating seasonal flu. So far tests on the April 11 CDC isolate show that it is sensitive to both drugs but not to adamantanes, which are not often used because of widespread resistance.
As the CDC receives more isolates, it will conduct further antiviral sensitivity tests.
Earlier this week the CDC started shipping a new test kit for detecting the H7N9 virus. It got a head start on developing the diagnostic test using gene sequences that China uploaded to the Global Initiative on Sharing All Influenza Data (GISAID) database early in the outbreak. However, the CDC needed the actual H7N9 isolate to confirm the test's sensitivity and specificity.
The virus so far doesn't appear to spread easily from person to person, but health officials have warned about the possibility of imported cases. Earlier this month the CDC urged clinicians to watch for possible cases in the United States, especially in people getting sick after traveling to China or after exposure to someone infected with the virus.
The H7N9 isolate will also be used in lab studies to learn more about disease severity and pathology, according to the CDC. Meanwhile, other studies are slated to look at antibody response to the H7N9 virus, which will be useful for gauging population immunity. These tests are crucial for identifying the spectrum of the disease and determining whether mild or asympomatic cases are occurring alongside the severe cases.
"These studies are just getting underway; results are not expected for some time," the CDC said.
The antibody tests will also come in useful during candidate H7 vaccine virus development, the CDC said, adding that progress is continuing on the vaccine virus, in case one is needed.
Commenting on the latest H7N9 developments in China, the CDC said that most infections have been severe and that China is reportedly using oseltamivir to treatpatients and recommending the agent as prophylactic treatment for patients' close contacts who have symptoms.
The latest information suggests that many infected patients had contact with poultry or poultry environments; infected birds can shed a lot of virus in their mucus or droppings, the CDC said. People in poultry settings can become infected if they touch an infected bird or a contaminated environment, then touch their eyes, nose, or mouth. The virus can also pose an airborne risk when infected birds flap their wings, the agency added.
See also:
Apr 26 CDC H7N9 update