The Centers for Disease Control and Prevention (CDC) last night released a detailed analysis of H5N1 avian flu samples taken from a patient in Texas who was exposed to sick cows, which suggests that the infection might involve the eyes but perhaps not the upper respiratory tract.
Also, when CDC scientists compared the human H5N1 samples to viruses from cattle, wild birds, and poultry, they found in the human sample a mutation with known links to host adaptation.
Meanwhile, the US Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) announced that highly pathogenic avian flu has been confirmed in a cow herd from Idaho and Ohio agriculture officials said tests have confirmed the virus in a dairy herd that had received cows from Texas.
Sequencing shows adaptation change, but not increased transmissibility
The CDC detailed its sequencing findings, along with its observations about what those findings might mean for the clinical picture, in a technical update on its website. Scientists saw minor differences between the human specimens and the cattle samples and that the viruses had avian characteristics.
However, the human samples had one change—PB2 E67K—that has a known link to virus adaptation to mammalian hosts, seen before in people and animals infected with H5N1, as well as other avian flu viruses. Scientists emphasized that the marker hasn't been linked to transmission and that the results of the analysis doesn't change the CDC's assessment for H5N1 clade 2.3.4.4b viruses that the overall risk to human health remains low.
This highlights the value of increased genomic surveillance.
Raj Rajnarayanan, MSc, PhD, a computational biologist with the New York Institute of Technology, on X (formerly known as Twitter) said PB2 E67K alone isn't sufficient to enable efficient human to human transmission. "However, this highlights the value of increased genomic surveillance and rapid dissemination of sequence data ASAP."
Patient swabs didn't show upper respiratory involvement
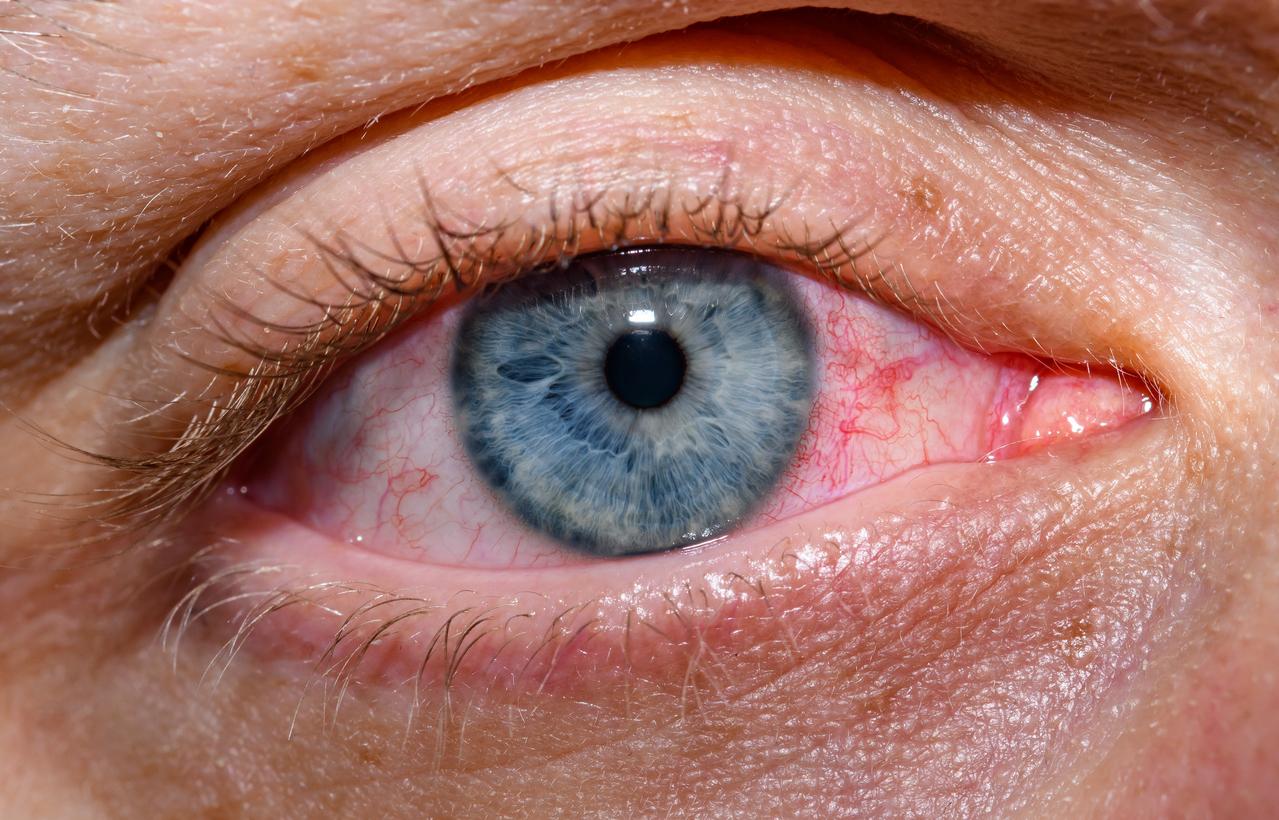
Also, the CDC detailed what investigators found during testing of the patient's nasopharyngeal and eye swabs. According to earlier reports, the patient's only symptom was conjunctivitis, a mild symptom seen in some earlier avian flu infections.
Testing on the nasopharyngeal sample didn't yield enough RNA for sequencing, but CDC scientists were able to sequence material from the eye swab sample.
"Notably, the patient reported only conjunctivitis with no respiratory or other symptoms, which likely resulted in lower viral RNA concentrations detected in the nasopharyngeal sample and is suggestive of a lack of respiratory infection in the patient," the CDC said.
Angela Rasmussen, PhD, a virologist with the University of Saskatchewan, on X said that it's good that the patient in Texas didn't become seriously ill, but she said a mild symptom like conjunctivitis may have a down side if mild cases aren't recognized and isolated.
We don’t want to give H5N1 the opportunity to adapt to efficient growth in humans. It can be deadly.
She warned that undetected cases gives the virus more opportunities to adapt to human hosts, underscoring the importance improved surveillance to identify infections in cows and humans and prevent new ones from occurring, along with reducing exposure risk.
"We don’t want to give H5N1 the opportunity to adapt to efficient growth in humans. It can be deadly," Rasmussen said. "To prevent the public health crisis of tomorrow, solve the problem of today."
Promising hints for vaccine and antiviral treatments
CDC scientists also looked at how well the hemagglutinin (HA) gene from the human specimen aligns with two candidate vaccine virus (CVV) strains it had already prepared for vaccine makers to make a vaccine, if needed. It said the HA from the human virus is very closely to the HA of both CVVs, which suggest that the vaccines would likely protect against the virus.
And finally, scientists looked at how well the virus from the human specimen might react to antivirals. The neuraminidase (NA) gene didn't have any resistance markers, which bodes well for the use of neuraminidase inhibitors like oseltamivir. They also examined other gene segments and found no resistance markers to antivirals that target the PA segment (baloxavir) or M2 (amantadine, rimantadine).
Confirmation in Idaho and Ohio herds
In other developments yesterday, APHIS said testing at the USDA's National Veterinary Services Laboratory (NVSL) in Ames, Iowa, has now confirmed a presumptive positive for highly pathogenic avian influenza in the dairy herd from Idaho. Earlier reports said the facility had received cows from another earlier affected state.
Today the Ohio Department of Agriculture announced NVSL has confirmed the virus in a dairy herd in Wood County, the state's first detection. It said the farm, located in the northwestern part of the state, on March 8 had received cows from a Texas far where the virus was later confirmed.
It added that the Ohio cows were tested after showing illness signs similar to those in other affected states.
APHIS also noted confirmation is also pending on more presumptive positive results from Texas, Kansas, and New Mexico.