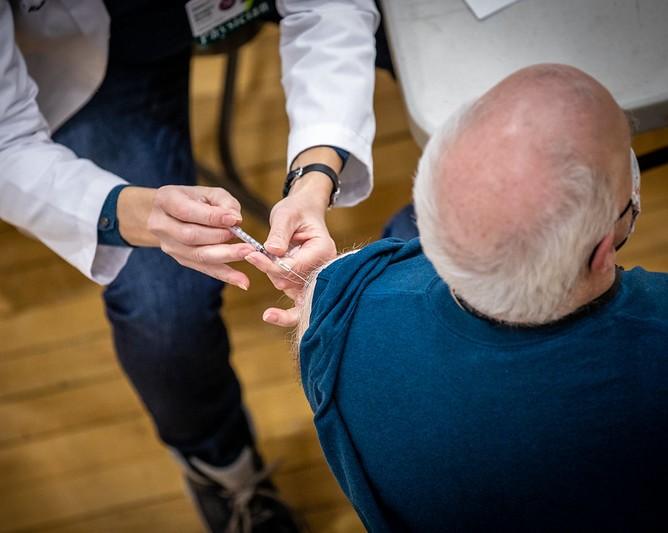
Centers for Disease Control and Prevention (CDC) vaccine advisers today recommended the Novavax COVID-19 vaccine for the primary series in adults, which comes as two more transmissible Omicron subvariants expanded their dominance to more than 90% of sequenced specimens.
Meanwhile, a top European health official today warned countries to take steps now to battle rising COVID levels, which could complicate efforts to battle a potential flu wave in the fall.
Another option for the unvaccinated
The Advisory Committee on Immunization Practices (ACIP), after presentations from federal health officials and Novavax and the group's own deliberations, unanimously approved the recommendation for people ages 18 years and older.
After the vote, Oliver Brooks, MD, chief medical officer with Watts Healthcare Corporation, said the primary vaccination target should be the 10% to 13% of the US population that remains unvaccinated. "We need to focus," he said. ACIP members welcomed the availability of a fourth COVID-19 vaccine and said they eagerly anticipate a plan for Novavax boosters in the near future.
Officials hope Novavax, made with more traditional cell-based processes, will sway some people to become vaccinated. It contains the SARS-CoV-2 spike protein produced in insect cells and contains the Matrix-M adjuvant to boost the immune system.
The Biden administration has bought 3.5 million doses of the vaccine, which is administered in a two-dose series given 3 to 8 weeks apart. Those with moderate or severe immune compromise should receive the doses 3 weeks apart.
Shipments of the vaccine have already begun, but providers can't administer it until the CDC accepts the ACIP's recommendation, which it typically does.
Monkeypox vaccine coadministration, other complexities
During the information part of today's presentation, CDC officials addressed coadministration of Novavax and other COVID-19 vaccines with orthopox vaccines, given the expanding rollout of the monkeypox vaccine as a key part of the response to that outbreak.
They said if orthopox vaccine is given first, healthcare providers should consider waiting 4 weeks before administering COVID-19 vaccine. And if Novavax or another COVID-19 vaccine is given first, orthopox vaccination should not be delayed.
Officials also covered a few benefits and drawbacks providers will encounter. Though the vaccine can be kept under normal refrigeration and doesn't need to be diluted, the 10-dose multidose vials expire 6 hours after opening, which could contribute to wasted doses. Also, the expiration date isn't on the vial or box, meaning healthcare workers will have to check them on a web-based portal.
During today's discussions, several ACIP members aired frustrations about the lack of expiration dates and other labeling inconsistencies, which they say pose an extra burden on already-stressed health workers.
Also, several members pressed CDC officials for more guidance on boosters, especially amid a growing BA.5 wave. They also asked about when to administer extra shots, given uncertainties on when the new bivalent boosters will be available in the fall.
BA.4 and BA.5 cause 90% of cases
Meanwhile, the CDC said today that BA.4 and BA.5 subvariants last week made up slightly more than 90% of sequenced samples, up from about 81% the week before. The gain was solely due to BA.5, which rose from 68.7% to 77.9% of samples.
BA.4 declined slightly from 14.4% to 12.8% of samples. Both are more transmissible than earlier Omicron subvariants, and both subvariants have mutations linked to escape from immune protection from earlier infection and vaccines.
As overall US cases continue a slow but steady rise, children were among the group to see an increase in infections, the American Academy of Pediatrics said its weekly update. More than 75,000 child infections were reported for the week ending Jul 14, up from 68,000 the week before.
In other US developments, the CDC said it has stopped reporting COVID levels for cruise ships in US waters, now that the cruise industry has the tools to mitigate COVID on passenger ships, according to the Washington Post.
European case rise is Delta déjà vu
With rising COVID cases in Europe, with cases tripling over the past 6 weeks, the continent is in a similar situation to last year at this time, when the Delta variant swept across the region, Hans Henri Kluge, MD, MPH, director of the World Health Organization (WHO) European regional office, said today. He said in both instances, the spread came amid relaxed restrictions and more social mixing.
He warned that hospitalizations are starting to rise and are expected to increase further in autumn and winter as schools reopen and people move indoors. "This forecast presents a huge challenge to the health workforce in country after country, already under enormous pressure from dealing with unrelenting crises since 2020," Kluge said.
He warned governments that the time to act is now, warning that the Southern Hemisphere in its winter season is battling a very active flu season alongside COVID-19—a situation that could also play out in the Northern Hemisphere. Kluge urged countries to strengthen COVID-19 surveillance as part of wider-based efforts for tracking a range of respiratory viruses.
In a related development, the WHO's European office today posted guidance for managing COVID and other respiratory viruses in the upcoming fall and winter, which focuses on protecting the most vulnerable groups.