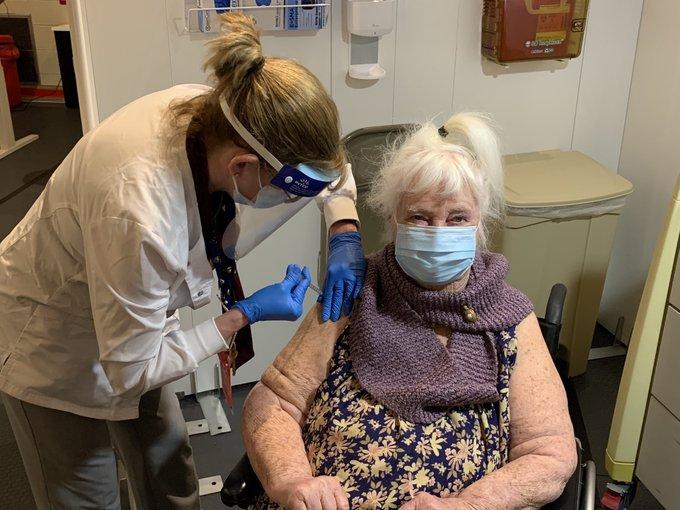
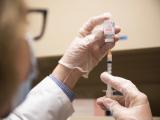
States excitedly announced the arrival of their first COVID-19 vaccine shipments today, as the first Americans, mostly healthcare workers, appeared in front of cameras to receive the first doses on a day that the nation passed the grim threshold of 300,000 deaths.
Developments moved quickly over the weekend with actions by federal agencies. The Food and Drug Administration (FDA) formally approved emergency use for the Pfizer-BioNTech vaccine the evening of Dec 11.
And yesterday the Centers for Disease Control and Prevention (CDC) accepted the Advisory Committee on Immunization Practices (ACIP) recommendation that people ages 16 years and older should receive it, with health workers and nursing home residents immunized first. The CDC detailed the ACIP recommendations in an early release Morbidity and Mortality Weekly Report document.
Immunization begins in priority groups
Soon after the two federal agencies jumped those last two hurdles, trucks yesterday began hauling shipments of vaccine from Pfizer's facility near Kalamazoo, Mich., to a nearby airport in Grand Rapids, where they were loaded onto planes for nationwide distribution.
This morning, a critical care nurse in New York named Sandra Lindsay is believed to be the first American to receive the vaccine outside of a clinical trial, according to the Washington Post, a scene repeated across several states and health facilities throughout the day. Sites included a Veterans Affairs (VA) hospital in Bedford, Massachusetts, which said Margaret Klessens, a 96-year-old World War 2 veteran who lives in a nursing home, was the first to receive it in a VA facility.
The White House scuttled an earlier plan for top government officials to receive the first doses, according to NPR.
Federal officials say the first shipments will be staggered, with vaccine arriving at 145 distribution centers today, 425 sites getting deliveries tomorrow, and the remaining 66 on Dec 16, according to the Associated Press.
Though nursing home residents are among the groups targeted to receive the first doses, only four states are ready to begin administering them to residents and staff, according to the Washington Post. In an update, Operation Warp Speed chief operating officer Gen. Gustave Perna didn't name the states that are ready, but said most won't begin until next week, because the immunizations can't be given until nursing home staff have obtained written consent from residents or their family members.
In other vaccine developments, the Trump Administration is rushing to roll out a $250 million public education campaign to encourage Americans to be vaccinated against COVID-19, according to the New York Times. It said the Department of Health and Human Services (HHS) is overseeing the campaign, which will include ads in print, social media, and radio formats.
And state leaders quoted in the Wall Street Journal said they are billions short in funding needed to roll out the COVID-19 vaccine to Americans who want to be immunized by June. They said additional funding is needed to hire medical workers, do community outreach, set up vaccination clinics, and ensure proper storage for the vaccines.
Deaths top 300,000
Yesterday, the nation added 1,389 deaths to its fatality total, and with more reported today, the overall number passed 300,000, according to the Johns Hopkins online dashboard. The nation crossed the 200,000 death threshold on Sep 22.
In its latest fatality projections, the CDC estimates that deaths will total between 332,000 and 362,000 by the week ending Jan 2.
In other developments:
- New York Gov. Andrew Cuomo said today that the state faces another shutdown if its COVID-19 patterns don't change, CNN reported. He said more efforts are needed to expand hospital capacity and that people need to do more to slow the spread of the virus in their homes, which he said accounts for 74% of transmission. On Dec 11, Cuomo suspended indoor dining for New York City starting today.
- A bipartisan Senate group is expected to unveil a $908 billion coronavirus relief package this week, according to Politico. The deal's components include $748 billion for schools and hospitals and a $160 billion piece that combines help for states and local governments and a temporary liability shield.
- FDA vaccine advisors will discuss Moderna's application for emergency use authorization (EUA) for its mRNA COVID-19 vaccine on Dec 17. The company said on Dec 11 that the US government has exercised an option to buy 100 million more doses of its vaccine, bringing the total order to 200 million doses.
- The US COVID-19 total has climbed to 16,388,504 confirmed cases, and 300,267 people have died from their infections, according to the Johns Hopkins tracker.