Outbreak responders in the Democratic Republic of Congo (DRC) are investigating five more suspected Ebola cases, as two more patients died from their infections and the World Health Organization (WHO) shared more details about how experimental treatments will be used and studied among those sickened by the virus.
The new suspected cases come as tests ruled out an earlier suspected case, and all are from known contacts, Peter Salama, MD, the WHO's deputy director-general of emergency response, said on Twitter. The developments raise the outbreak total to 58, which includes 37 confirmed, 14 probable, and 7 suspected cases. The two new fatalities lift the number of deaths to 27.
New treatment details
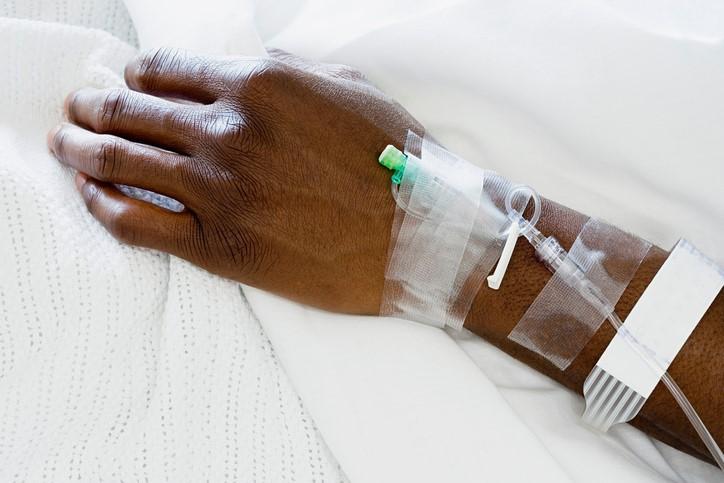
Earlier this week the WHO had signaled that a DRC health ministry ethics committee had approved the use of five experimental treatments for compassionate use, also known as Monitored Emergency Use of Unregistered Interventions (MEURI), and was finalizing details of a study protocol.
In a statement today, the WHO said clinicians working in the treatment centers will make decisions on which drug to use, based on what would be helpful for patients and what's appropriate for the setting. It noted that experimental treatments can be used as long as patients give informed consent and that treatment and monitoring protocols are closely followed.
The WHO also posted the details of an expert consultation held on May 17 to evaluate five therapies that could be used in the DRC's outbreak, along with considerations for their use. Four of the drugs are already in the DRC: ZMapp (a monoclonal antibody cocktail), Remdesivir (an antiviral also known as GS-5734), Regeneron (monoclonal antibody cocktail), and mAb114 (monoclonal antibody). The other drug the experts considered was favipiravir, an antiviral medication.
In weighing study data so far, the group said there is already high quality data for ZMapp, which has been studied in a randomized controlled trial of patients with Ebola. The panel said the benefits outweigh the risks of the drug.
For Remdesivir, the group said available data support its use, but concerted efforts should be made to study it in clinical trials to assess the risks and benefits for Ebola patients. Studies involving Regeneron look very promising and support compassionate use in settings where ZMapp and Remdesivir aren't available, the experts said.
Benefits of favipiravir aren't clear, and the group said it's important to establish if the antiviral is useful for Ebola patients. Experts said compassionate use of favipiravir could be considered when the other three experimental treatments aren't available. They also noted that use of the drug is complicated by dosing selection for Ebola treatment, though one advantage is that it can be orally administered.
In addition, though early data on mAb114 look promising, more are needed before recommending it for compassionate use, they said.
The team also looked at the challenges in using the drugs in the outbreak setting. For example, ZMapp needs to be refrigerated and requires adequate staffing because of the technical demands of administering the drug, which can require a long infusion time. For Remdisivir, lab values would have to be monitored, and it's unclear if that capacity exists in the field.
Point-of-entry response steps
In a separate report, the WHO yesterday said it, the health ministry, and response partners have developed a strategy for preventing the spread of Ebola to other provinces or countries by implementing health measures such as devising a risk communication strategy, providing hand hygiene, and developing illness alert procedures.
The agency said 115 entry or traveler congregation points have been identified in Mbandaka, Bikoro, Iboko, Equateur Province, and Kinshasa. Thirty of the locations have been prioritized for more in-depth assessment and screening, including major ports along the Congo River, two airports, and an international port in Kinshasa. Also included are areas where people gather, such as markets.
Entry and exit screening are already under way at the Mbandaka airport and in some terminals at Kinshasa's international airport.
White House reverses taking away Ebola funds
The Trump Administration yesterday removed cuts in Ebola funding, along with a few other items, from its proposed budget rescissions yesterday, Roll Call reported.
The Ebola money targeted for cuts last month was $252 million in unspent funds that the last administration had allocated for West Africa's Ebola outbreak, funding that was meant to help countries at risk for the disease improve their health and emergency response systems.
The originally proposed rescissions were first announced in early May, as news was emerging of the current DRC outbreak, for which the US Agency for International Development (USAID) has contributed up to $8 million. The Trump Administration has praised the DRC for allocating its own funds and other countries for their significant contributions to the response.
See also:
Peter Salama Twitter feed
Jun 6 WHO statement
Jun 5 WHO statement on entry points
Jun 5 Roll Call report