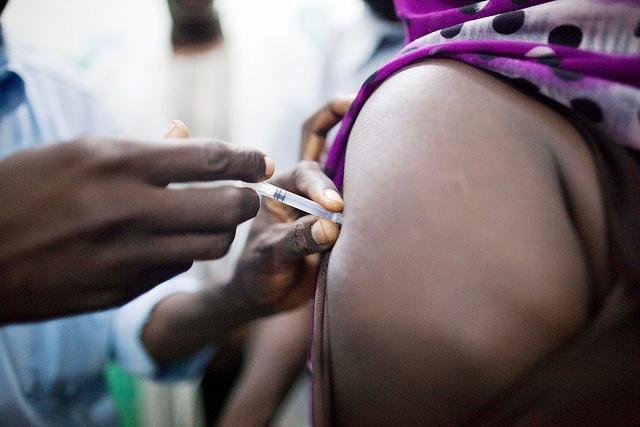
In an effort to see if a vaccine might be used to drown out Ebola flare-ups, a new trial launched in an area of Guinea where cases have occurred recently, while Chinese researchers reported the first human trial results for a different type of vector vaccine.
The trial in Guinea involves one of the two vaccines that are furthest along in clinical testing, while the vaccine in the Chinese study has been quickly adapted, with several research questions still to be settled before it proceeds to the next testing phase.
Ring strategy used for Guinea trial
The WHO said today that the first trial of an Ebola vaccine using the ring vaccination strategy launched in Guinea this week, with a team arriving in the small village of Basse-Guinee in Coyah district on Mar 23 to vaccinate contacts of recently infected people who have consented to participate. It added that vaccinations for now will include only adults, except for pregnant women.
Volunteers will receive the VSV-EBOV vaccine, developed by Canadian researchers and licensed by NewLink Genetics and Merck. It uses an Ebola virus protein spliced into a vesicular stomatitis virus (VSV).
Ring vaccination was used to eradicate smallpox in the 1970s, and the trial is designed to show if a similar strategy can help snuff out pockets of Ebola cases as they crop up. The trial design, put together by an international group of experts, also offers an alternative to using a placebo. According to the original plan, about 10,000 are to be vaccinated in 190 rings within a 6- to 8-week period. Volunteers will be followed for 3 months, with results available as early as July.
Jean-Marie Dangou, MD, the WHO's representative in Guinea, said in the statement, "The start of ring vaccination clinical testing in Guinea is therefore one of the most important milestones we have achieved in seeking a modern line of defense against Ebola."
The trial starting in Guinea is the second in the outbreak region for Ebola vaccines. A phase 2/3 trial of the ChAd3 vaccine, which uses a modified chimpanzee adenovirus vector, and VSV-EBOV launched in Liberia in early February. A phase 1 human trial launched in the United Kingdom in January for another vaccine combination, a prime-boost regimen from Johnson & Johnson and Bavarian Nordic, and Emergent Biosolutions recently announced that a trial of a boosted version of the ChAd3 vaccine could soon launch in the UK.
WHO vaccine experts have said different vaccines and different strategies may be needed to battle Ebola, and several research questions still need to be answered about which vaccine candidates are likely to offer the most doses, the quickest protection, and the longest-lasting protection.
However, declining Ebola levels in the three countries are making it difficult to assess if the vaccines will be effective in outbreak settings.
Phase 1 results for Chinese vaccine
In another vaccine development, a research team today reported the first phase 1 results for an Ebola vaccine developed in China, saying that a single high dose prompted a robust immune response in 14 days. The findings appear today in The Lancet. The Chinese vaccine is a recombinant adenovirus type-5 platform that encodes the glycoprotein from the 2014 West African Ebola virus. Though other vaccines in human trials have used genetic material from the Zaire strain, the Chinese vaccine is the only one that includes components of the current outbreak strain.
The randomized, double-blind, placebo-controlled trial took place in China's Jiangsu province and involved 120 adults ages 18 to 60 years. Groups of 40 received a placebo, a low dose of the vaccine, or a high dose.
Mild injection-site pain was the only adverse event and was reported in all three groups, with a higher incidence in the high-dose group. Immune response was significantly higher than placebo for both groups, with a magnitude that reflected the dose. Glycoprotein-specific antibodies increased by day 14 and continued to rise until day 28, and glycoprotein-specific T-cell response peaked on day 14.
However, the presence of preexisting immunity to adenovirus type-5 partly blunted both types of responses, especially in the low-dose group. The team said the findings suggest the high-dose version could overcome preexisting immunity. They added that the high dose would probably be needed, because many adults have preexisting adenovirus type 5 immunity, especially in developing countries. Because preexisting immunity baselines are likely to be even higher in African populations, the vaccine's immune response should be further assessed in Africa, they said.
The group said an important consideration for vaccine vector has been a type of CD4 T-cell activation that could increase rates of HIV-1 acquisition in those who have positive anti-adenovirus type-5 immunity, and though the association is controversial and the mechanism unclear, the potential risks should be considered, especially in Africa. They wrote that the number of participants in their study wasn't high enough to assess the risk, but participants will be monitored for at least 1 year.
More evidence is needed to assess whether a vaccine based on the 2014 West African Ebola strain performs better than ones based on the 1976 Zaire strain, the team wrote. The authors said the adenovirus type-5 vaccine is formulated as a lyophilized white powder, which might be more stable and easier to store and transport in settings that lack good cold-chain capacity, such as parts of Africa.
They concluded that the adenovirus type-5 Ebola vaccine has potential, but more research is needed to assess the HIV-1 and preexisting immunity issues. Though a one-dose approach might be simpler and more efficient, it might not be enough to provoke long-lasting immunity, and a prime-boost combination might be needed to provide broader or more durable immunity, the team wrote.
Findings raise new questions
In an accompanying editorial in The Lancet, two virologists wrote that preexisting immunity is a major issue concerning the vaccine platform, because 80% of the target population in Africa likely has neutralizing antibodies to adenovirus type-5. The authors are Andrea Marzi, PhD, with the National Institutes of Health's Rocky Mountain Laboratories in Hamilton, Mont., and Darryl Falzarano, PhD, with the University of Saskatchewan.
Marzi and Falzarano said they hope that data from animal studies will shed light on the importance of having a vaccine antigen that is identical to the circulating virus. They noted that since antibody response was followed only up to day 28, questions remain about the vaccine's durability and long-term side effects.
The Chinese team's experience with the adenovirus type-5 Ebola vaccine vector shows how quickly existing platforms can be retooled to incorporate a new virus strain and move to human trials, with minimum testing in animals, the pair said.
They wrote that one of the biggest questions is whether the Chinese vaccine platform, or any others that are being studied for Ebola, are more effective than VSV-based vaccines, which seem to act fast and aren't hampered by preexisting immunity to the vector.
See also:
Mar 25 WHO statement
Mar 25 Lancet abstract
Mar 25 Lancet editorial