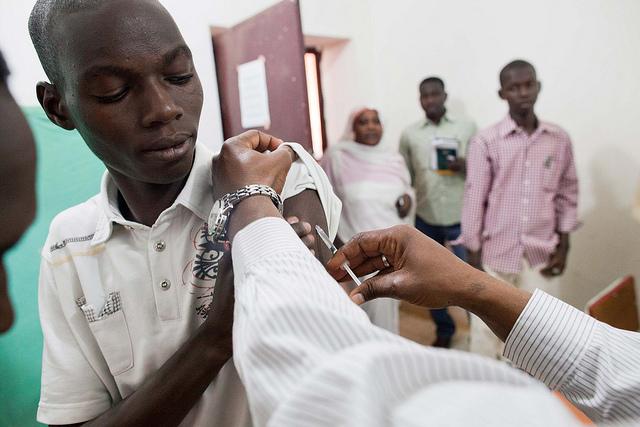
A clinical trial of two experimental Ebola vaccines was launched today near Liberia's capital, the first study to see if the vaccines are effective at reducing disease levels in the outbreak setting, where falling case numbers may thwart the ability to see any impact.
The phase 2/3 study, sponsored by the National Institutes of Health (NIH), is being conducted by a team of Liberian researchers and their colleagues from NIH's National Institute of Allergy and Infectious Diseases (NIAID).
Goal is 27,000 volunteers
Team members plan on enrolling 27,000 healthy adult volunteers in Montserrado County, which includes Monrovia, where social mobilization and engagement activities have already been under way to recruit study participants, the NIH said today in a press release. The group is also targeting those at higher risk for Ebola infections, including healthcare workers, communities with active transmission, contact tracers, and burial teams.
Volunteers in the randomized, double-blind study will receive one of two different vaccines or a placebo. The vaccines are ChAd3, which uses a chimpanzee adenovirus to deliver Ebola virus genetic material from the Zaire strain, and VSV-EBOV, which uses a vesicular stomatitis virus to deliver an Ebola gene segment.
ChAd3 was developed by the NIH and GlaxoSmith Kline, and VSV-EBOV was developed by the Canadian government and licensed to NewLink Genetics and Merck. The NIH has supported the development of both vaccines.
Study participants will be advised on how to minimize their risk of contracting Ebola infection and will be contacted by staff 1 week after injection, then monthly for up to 12 months.
A supply of vaccine has been made and initial safety studies have been conducted with unprecedented speed. But infection levels are falling quickly in all three of the outbreak countries, which will make it hard for researchers to measure any impact.
The NIH said in its statement that given the decline in cases, the team expects it will need to be flexible with the trial and its design.
Case decline hamstrings antiviral trial
In a related development, the maker of a drug being tested in Liberia as a possible treatment for Ebola has pulled out of the study because of the drop in cases, making it difficult for the trial to proceed. In a Jan 30 statement, Chimerix, developer of the antiviral drug brincidofovir, said it would stop participation in a study announced in December for Liberia as well as a supportive phase 2 study,
Though the drug was developed for treating other diseases, including adenovirus and cytomegalovirus infections, in vitro studies have hinted that brincidofovir may have activity against the Ebola virus. Researchers already had an idea of its safety profile from earlier clinical trials.
A handful of people who were sickened in the outbreak and airlifted out of the region have been treated with the drug, alongside supportive therapy and sometimes other experimental treatments.
A University of Oxford team had launched a study of the drug at a Doctors without Borders (MSF) treatment center in Monrovia on Jan 2.
Chimerix noted in its statement that over the last several weeks the number of new Ebola cases in Liberia has dropped significantly, with only a handful of patients enrolled in the single-arm study.
M. Michele Berrey, MD, MPH, the company's president and chief executive officer, in the company statement praised the research teams and commended the progress made in controlling the disease in Liberia. She said the company will move forward with developing the drug for preventing infections in transplant recipients and immunocompromised patients.
Case count rises slowly
In outbreak developments, the number of Ebola infections and deaths continued to rise slowly in all three countries, with confirmed, probable, and suspected cases reaching 22,334, including 8,921 deaths, the World Health Organization (WHO) said today.
Since the last report on Jan 30, the three countries have reported 208 confirmed, probable, or suspected cases, as well as 92 more deaths. Today's numbers reflect cases reported as of Jan 31 in Guinea and Sierra Leone and as of Jan 28 in Liberia.
Sierra Leone reported the largest number of new cases, 146, including 58 deaths, the WHO said.
MSF tracks gaps and hot spots
Meanwhile, MSF said in its latest update on Jan 30 that only 50 patients are in its treatment facilities, thanks to declining disease levels in the region. Though it called the development promising, it echoed the WHO's concern that only about half of new cases in Guinea and Liberia are in known contacts, which shows gaps in tracing needed to identify and address all chains of transmission.
MSF also aired concerns about a lack of information sharing on contacts among the three outbreak countries. "Since a single new case is enough to reignite an outbreak, the level of vigilance should remain high in order not to jeopardize the progress made in stemming the epidemic," the group said.
In Guinea, though cases have declined, the epidemic is still spread over a wide area—13 different districts—with new transmission chains reported from areas thought to be free of the virus, MSF said.
The group said its rapid response team is in Faranah, where new cases have been reported, and that a second response team is due in the area soon to help it address other transmission sparks.
Liberia's declining cases have prompted a scale-back in MSF activities, and though Sierra Leone is also seeing ongoing drops in new cases, the region still has about 50 hot spots, and underreporting is still a problem, the group said.
Ebola exposures prompt two UK airlifts
In other developments, two healthcare workers have been airlifted to the United Kingdom for isolation and observation after possible Ebola exposure while working in Sierra Leone, Public Health England (PHE) said in statements on Jan 31 and today. Both are military health workers, and both sustained needle-stick injuries.
One of the workers arrived in London on Jan 31 and was taken to the Royal Free Hospital. PHE said that though the patient was exposed to Ebola, he or she hasn't had any symptoms and hasn't been diagnosed with the disease. It added that the patient will be monitored for the 21-day incubation period.
The other health worker arrived in the UK today and was also taken to Royal Free Hospital for assessment. PHE said the patient isn't symptomatic and so far does not have an Ebola infection.
Mark Francois, minister for the Armed Forces, said in the PHE statement that though the two exposures occurred in a short time interval, they appear to be unrelated.
See also:
Feb 2 NIH press release
Jan 30 Chimerix press release
Feb 2 WHO situation update
Jan 30 MSF statement
Jan 31 PHE statement
Feb 2 PHE statement