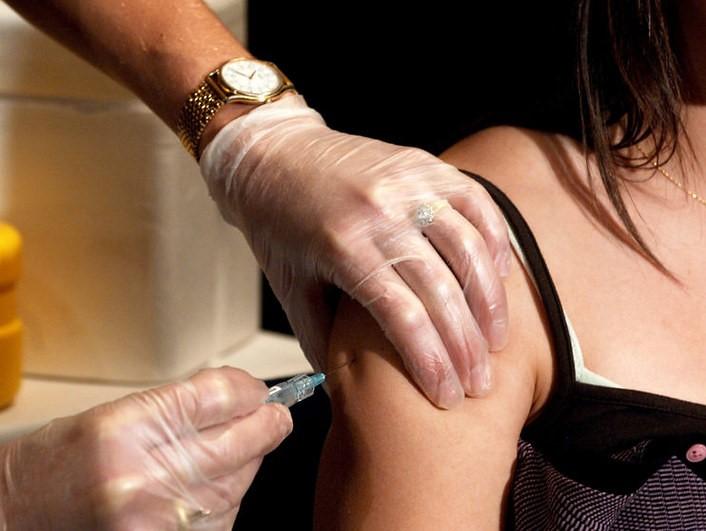
In a move poised to bring a second COVID-19 vaccine to the market for US kids, the Food and Drug Administration (FDA) advisory committee today approved the Moderna vaccine for children ages 6 through 17 years.
The vote by the FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) today precedes its deliberations tomorrow on EUA modifications for Moderna and Pfizer-BioNTech mRNA vaccines for the youngest children yet to receive vaccine eligibility, those ages 6 months to age 5. If approved, nearly all Americans would finally be eligible for COVID-19 vaccination.
The votes this week come as more parts of the country move into the high community-transmission category and more transmissible BA.4 and BA.5 Omicron subvariants expand their reach.
Concerns about Omicron protection
During today's discussions, VRBPAC weighed the benefits and risks of the two-shot vaccine series at a dose based on age: the 100-micogram adult dose for adolescents ages 12 through 17 and a 50-microgram dose for children ages 6 through 11. The votes passed unanimously for use of the vaccine in both age groups.
During the discussions, many members said they were comfortable with the data on the vaccine, and that approval of a second mRNA vaccine for kids provides parents another choice and greater access. They raised concerns about very rare myocarditis complications associated with mRNA vaccines, but said so far, they appear to be less common in adolescent males than in adult males.
However, some members raised concerns about how well a two-dose series will protect against Omicron, though a third dose wasn't an official part of the discussion.
Paul Offit, MD, professor of pediatrics at the Children's Hospital of Philadelphia, said with the Omicron variant, the United States is at a different stage of the pandemic than when the Pfizer-BioNTech vaccine was cleared for emergency use in kids at a two-dose series. Federal officials have since OKd use of Pfizer booster doses in younger age groups.
Offit said a third dose of Moderna should be part of the primary series against Omicron, and he asked CDC members of the group to use their influence with getting the message across that two doses shouldn't be considered fully vaccinated.
In the next steps, the FDA needs to decide whether to accept VRBPAC recommendations, as it typically does. Before doses are administered, the Centers for Disease Control and Prevention (CDC) vaccine advisors must make a formal recommendation. The group, the Advisory Committee on Immunization Practices (ACIP) meets on Jun 17 and Jun 18.
High community spread as subvariants make headway
The nation's COVID-19 activity continues to rise and evolve, partly driven by the arrival of BA.4 and BA.5, two Omicron subvariants that are known to have a higher growth rate.
Yesterday CDC Director Rochelle Walensky, MD, said on Twitter that 67% of the US population currently live in areas with medium or high community transmission, up from 55% last week.
In its weekly update on variant and subvariant activity, the CDC said BA.5 now makes up 13.3% of new sequenced samples, up from 7.7% last week. Also, BA.4 now makes up 8.3% of new cases, up from 5.4% the week before. Some parts of the country are feeling an even greater impact from the subvariants, such as one of the southern regions where they make up 30% of new cases. The region includes Texas, Oklahoma, Arkansas, Louisiana, and New Mexico.
Alongside transmissibility concerns, early findings suggest the two subvariants have mutations linked to immune escape, which would reduce some of the protection from earlier illness, vaccines, and even treatments.
Also, about 88,000 pediatric COVID-19 cases were reported last week, about the same as the week before, according to the latest update from the American Academy of Pediatrics, which said new cases in children continue to decline following Omicron's peak.