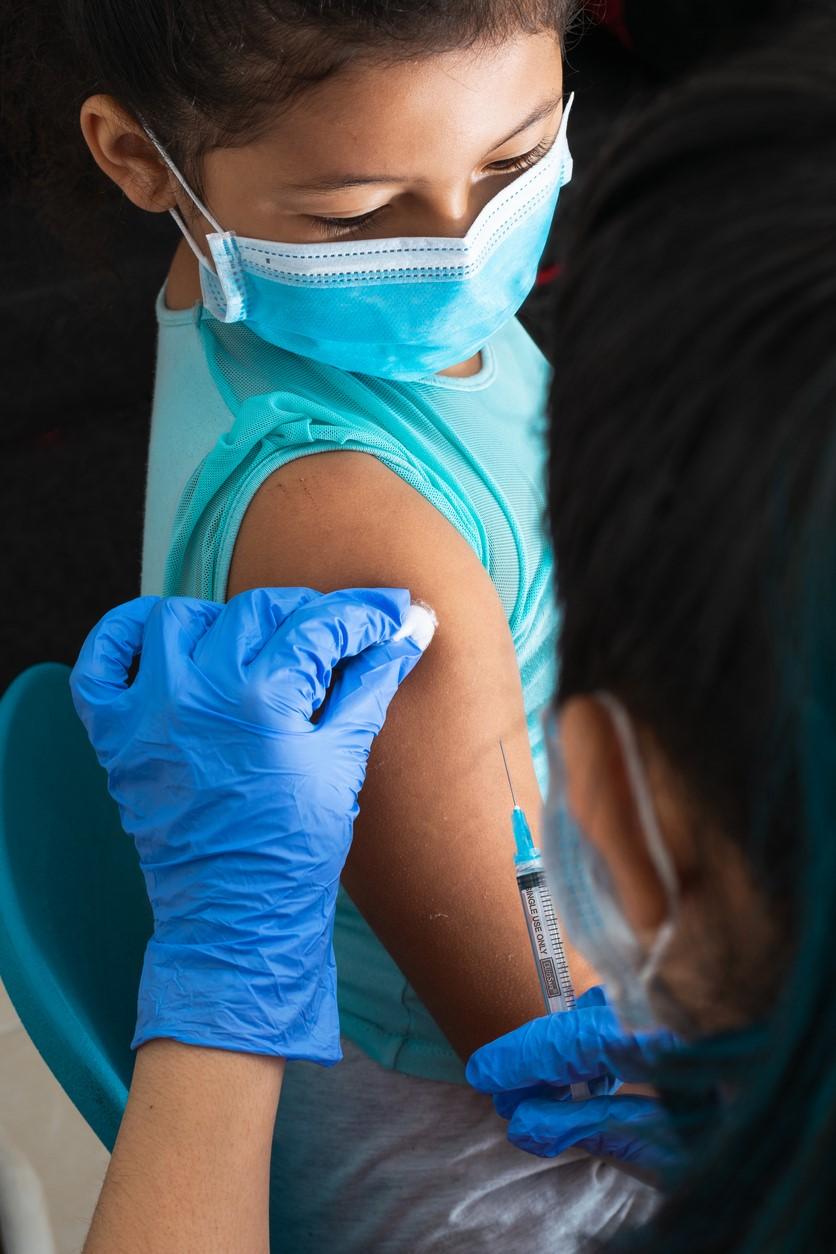
Following the lead of its advisers, the US Food and Drug Administration (FDA) today granted emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for children 5 to 11 years old.
The step moves vaccination of that age-group a step closer to becoming a reality. The Advisory Committee on Immunization Practices (ACIP), which reports to the Centers for Disease Control and Prevention (CDC), will take up the matter on Nov 2. The CDC typically follows ACIP advice, and once it clears the vaccine, it will roll out to younger kids across the country.
States are preparing to receive hundreds of thousands of doses of the lower-dose Pfizer vaccine for young children as early as next week.
Three days ago, the 19-member Vaccines and Related Biological Products Advisory Committee, which advises the FDA, voted overwhelmingly to support vaccination of young children, saying that the benefits outweigh the risks.
Data show 91% vaccine effectiveness
"As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today's authorization,” said Acting FDA Commissioner Janet Woodcock, MD, in an FDA news release.
"Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy. Our comprehensive and rigorous evaluation of the data pertaining to the vaccine's safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards."
The EUA is based on a "thorough and transparent" assessment of the data, including studies that show the Pfizer vaccine provides 90.7% protection against COVID-19 in children 5 to 11 years old—comparable to vaccine effectiveness in teens and young adults. Safety was assessed in about 3,100 children 5 to 11 years old.
Children that age account for 39% of COVID cases among all children, the FDA said, and about 8,300 of them have been hospitalized. As of Oct 17, COVID-19 deaths in 5- to 11-year-olds totaled 146, and 691 children of any age have died from the coronavirus.
If approved by the CDC, the Pfizer vaccine will be administered as a two-dose series 3 weeks apart. Each shot will include 10 micrograms of active ingredient, compared with 30 micrograms in older children and adults.
Weighing the evidence
In the release, the FDA said, "Based on the totality of scientific evidence available, the known and potential benefits of the Pfizer-BioNTech COVID-19 vaccine in individuals down to 5 years of age outweigh the known and potential risks."
"The FDA is committed to making decisions that are guided by science that the public and healthcare community can trust," said Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research. "We are confident in the safety, effectiveness and manufacturing data behind this authorization."
The effectiveness data to support the EUA in younger children come from an ongoing randomized, placebo-controlled study that has enrolled roughly 4,700 children 5 through 11 years in the United States, Finland, Poland, and Spain. In that study, immune responses in this age-group were comparable to those of older children.
The FDA also analyzed COVID-19 cases that occurred 7 days after the second vaccine dose. Those data showed 3 cases of COVID-19 among 1,305 vaccine recipients and 16 cases in 663 children who got a placebo, which translates to 90.7% effectiveness.
The agency also said no concerning side effects were found. It added that Pfizer has updated its safety monitoring plan to include evaluation of myocarditis (inflammation of the heart muscle), pericarditis (inflammation of the lining of the heart), and other events. The heart-related events have occurred rarely in older children, but the FDA's modeling research revealed that the benefits of vaccination outweigh the risk in the younger age-group
The FDA added, "In addition, the FDA and the CDC have several systems in place to continually monitor COVID-19 vaccine safety and allow for the rapid detection and investigation of potential safety problems."
Formulation change to improve storage
The FDA today also authorized a manufacturing change for the Pfizer COVID vaccine to include a formulation that uses a different buffer. The new formulation is more stable for longer during refrigeration, providing greater flexibility for vaccinators.
The new formulation contains Tris buffer, which is commonly used in other FDA-approved vaccines and other biologics, including products for children. The FDA said Tris buffer doesn't present safety or effectiveness concerns.
See also:
Oct 26 CIDRAP News story "FDA panel green-lights Pfizer COVID vaccine for young kids"