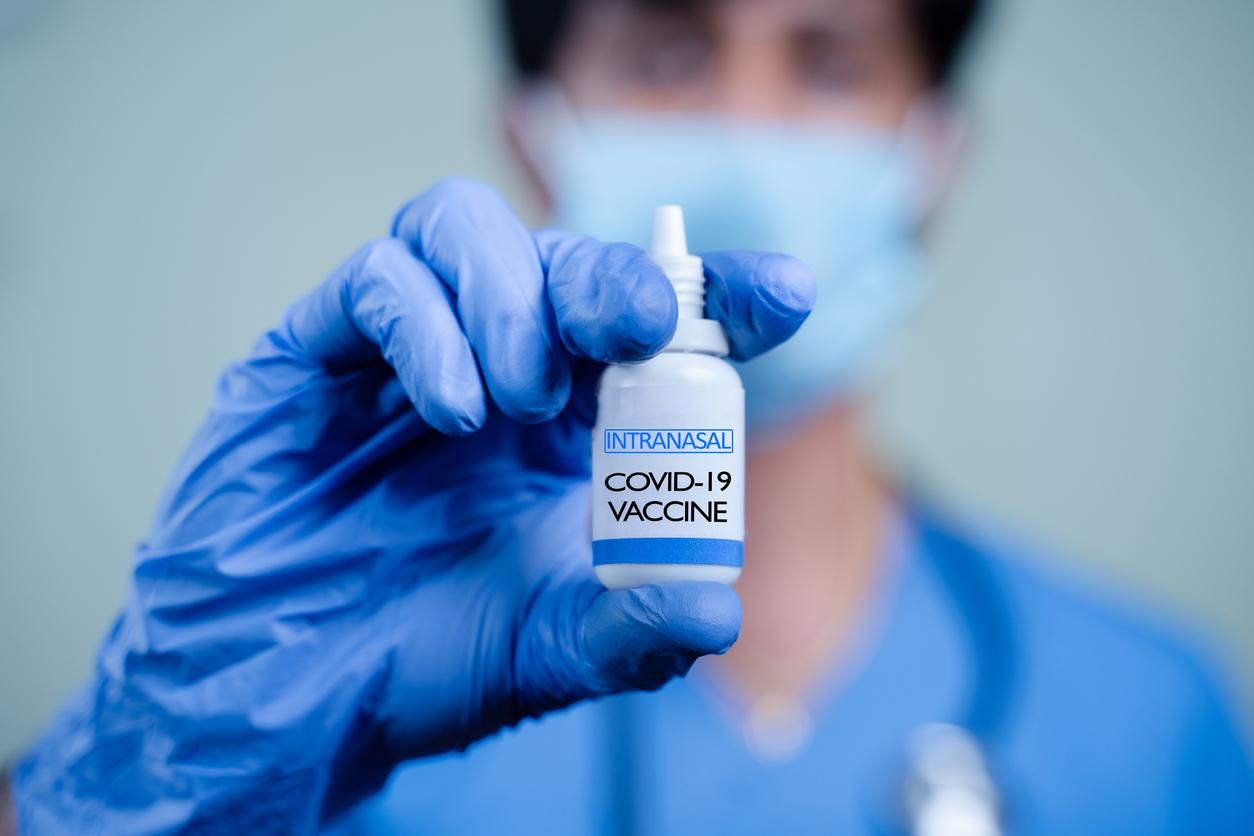
As the nation grapples with surges of Omicron subvariants that evade immunity from vaccination and previous infection, officials and pharmaceutical companies are starting talks on how to improve the shots and make them easier to administer.
In a related development, federal officials have signaled that second boosters for those under 50 are on hold to speed production of new bivalent shots.
Wish list: broader protection, easier administration
The White House tomorrow will host a summit on the future of COVID-19 vaccines, which will be streamed online. One of the main topics is speeding development of a more broadly protective COVID-19 vaccine.
The Food and Drug Administration (FDA) vaccine advisory group recently recommended a bivalent booster shot that includes the original SARS-CoV-2 strain and an Omicron variant, and at the meeting, several members aired concerns that officials will more frequently face the challenge of tweaking the vaccine to keep up with the quickly evolving virus.
At the global level, researchers are working on a roadmap for developing a new coronavirus vaccine to broadly protect against the most dangerous ones.
At tomorrow's summit, experts will also discuss new options for delivering the vaccines, especially ways to make them easier to administer, such as intranasal or patch formulations. Participants will also tackle vaccine equity at both the domestic and global levels.
Second booster holding pattern for under-50 adults
As the BA.5 variant fuels outbreaks in several parts of the country, with hospitalizations also on the rise, federal officials have urged eligible groups to get their booster doses, especially those over 50 who are recommended to get a second booster. However, officials still haven't recommended second boosters for adults younger than 50.
Health officials are waiting to see if vaccine makers can more quickly deliver the updated boosters, by the middle of September, unnamed sources told the Washington Post. At a recent FDA advisory committee meeting, company officials said the updated boosters might be ready only in October.
In other COVID developments:
- President Joe Biden's COVID-19 symptoms have almost completely resolved, except for residual nasal congestion and minimal hoarseness, his doctor, Keven O'Connor, DO, said today. Biden completed his fourth full day of Paxlovid treatment and is working remotely.
- US COVID-19 patterns are in flux, with new daily cases down 2% last week, hospitalizations up 4%, and deaths level, according to the Washington Post Health officials have cautioned that it's difficult to gauge trends due to scaled-back testing policies and increased use of home-based rapid tests.
- Nearly 87% of the US population lives in locations with medium to high COVID community levels, the US Centers for Disease Control and Prevention (CDC) said in a Jul 22 update.
- In the European region, COVID-19 cases declined slightly last week, by 4.4%, but deaths rose 8.8%, with about 90% of the deaths in those ages 65 and older, according to the latest surveillance update from the European Centre for Disease Prevention and Control (ECDC). Pooled hospitalizations and intensive care unit (ICU) admission rates were up slightly.