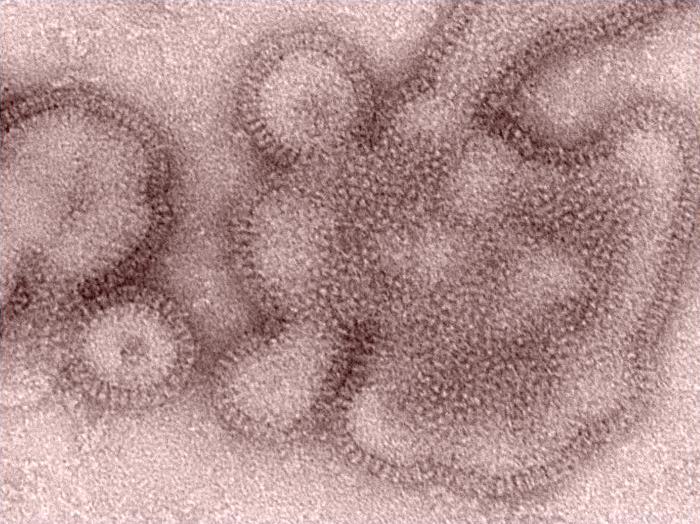
Influenza experts were puzzled last year when the seasonal flu vaccine didn't work very well against H3N2 flu viruses, the dominant strain at the time, even though the vaccine was said to be an excellent match for the viruses going around.
Now a large team of Canadian scientists thinks it has an explanation for the problem: Mutations occurred when the H3N2 reference strain recommended for use in the vaccine was adapted to make it grow well in eggs.
Those mutations weakened the immune response to the A/H3N2 component of the vaccine, contends the team, led by Danuta Skowronski, MD, lead epidemiologist with the Influenza and Emerging Respiratory Pathogens branch of the British Columbia Centre for Disease Control.
"These mutations acquired with egg adaptation have been recognized for decades, and have been described," Skowronski said in an interview. "But our study is the first to show the epidemiologic impact of those mutations in people." The study was published this week in PLoS One.
World Health Organization (WHO) flu experts noticed the mutations in the egg-adapted strain and recommended using a different egg-adapted virus for this season, without changing the original reference strain. But Skowronski's findings suggest that the replacement strain also may lead to low vaccine effectiveness. In the current season, relatively few H3N2 flu cases have been detected in North America, making it hard to assess vaccine effectiveness (VE) against H3N2.
Other experts have greeted the findings with a mix of reactions. Nancy Cox, PhD, a top flu expert at the US Centers for Disease Control and Prevention (CDC), cautioned that the study shows that the mutations are associated with reduced VE but don't prove that they are the cause. Others called the findings an important contribution that underscores the need for better flu vaccines and production technology.
Good match, low effectiveness
Skowronski and her colleagues at institutes across Canada used a test-negative case-control study to monitor flu VE in 2012-13. The method involves testing for flu in patients who seek medical care for flu-like illnesses and determining their vaccination status.
They found that the vaccine was 50% effective overall, but only 41% effective against H3N2, which accounted for most cases that season. VE against the 2009 H1N1 virus was 59% and against influenza B was 68%. They note that there were similar findings in the United States.
What was puzzling was that analyses of circulating H3N2 flu viruses showed that they were well-matched to the prototype H3N2 strain recommended by the WHO for use in the vaccine. Circulating flu viruses often acquire mutations, a process called antigenic drift, which tend to reduce the effectiveness of flu vaccines by making the viruses less recognizable to an immune system primed by vaccination or prior infection.
Continuous antigenic drift is the reason flu vaccines are reformulated each year.
"We were reporting low vaccine effectiveness [against H3N2] at the same time that every lab around the globe was reporting that circulating viruses were antigenically similar to the vaccine. They had not evolved," Skowronski said.
Adding to the mystery was that Canada was seeing the most flu outbreaks in long-term care facilities in a decade, despite high immunization coverage among residents and staff, she said.
Reassortment in the lab
Most of the world's flu vaccine supply is still produced by growing the target viruses in chicken eggs. But laboratory manipulations are necessary to obtain a strain that will grow well in eggs. Typically this involves joining the hemagglutinin (HA) and neuramidase (NA) genes of the target virus, such as H3N2, to a backbone of six genes from a laboratory strain that's known to thrive in eggs. HA and NA stud the virus's surface and are visible to the immune system.
In the lab procedures, random mutations in the target virus elements can arise, Skowronski notes. "To adapt to an avian host, the virus has to acquire certain mutations," she said. "They represent a tradeoff between a virus that's antigenically conserved and one that can nevertheless replicate in avian cells. And those aren't necessarily mutations that we want."
Aware of these possible mutations, she said, "We set out to compare our circulating viruses not just to the cell-passaged version of the [WHO-recommended] reference virus but also to the egg-adapted version, recognizing that what's in the vaccine is not the cell-passaged but the egg-adapted virus."
She explained that the usual way to test whether viruses are antigenically related is to infect ferrets and see if the antibodies they produce in response to one virus inhibit another to a similar level in a hemagglutination inhibition (HI) assay. If there is an eightfold or greater difference in HI antibody levels produced to one virus when tested against another, the two viruses are considered antigenically distinct.
Using HI, the team showed that ferret antibodies produced to the WHO reference virus inhibited H3N2 viruses collected from patients at the same HI antibody levels, meaning they were antigenically equivalent, she said.
However, ferret antibodies produced to the egg-adapted version of the reference strain inhibited all but one of 132 circulating viruses at HI levels that were at least eightfold lower compared with the egg-adapted virus. This reduction in HI antibody titer was also seen when the ferret antibodies to the egg-adapted strain were tested against the WHO reference virus.
Together these findings indicated that the egg-adapted strain differed from circulating viruses and also from the strain originally recommended by the WHO.
Genetic sequencing findings
To look for possible causes of these antibody differences, the researchers sequenced the HA gene of H3N2 viruses collected from patients and compared those sequences with the WHO reference virus, called A/Victoria/361/2011, and also to the egg-adapted strain, called IVR-165.
They found three mutations at antigenic sites of the vaccine strain that differed from circulating viruses and from the WHO reference strain. Sequencing of 152 circulating H3N2 viruses from flu patients revealed no mutations at those three sites, which remained matched to the reference virus.
"We learned that it wasn't because of something happening in nature, but rather something in manufacturing [that caused the mutations]. It was actually at the vaccine candidate virus stage, the egg-adapted stage, that those mutations in the vaccine virus were introduced," Skowronski said.
The report sums it up: "Through detailed gene sequencing and HI comparison we show that reduced vaccine protection during the 2012-13 season was related to mutations in the egg-adapted H3N2 high growth reassortant strain used in vaccine production, not antigenic drift in circulating viruses."
The findings echo results of animal research, according to the report. In various animals, H1 and H3 viruses derived from mammalian cells have been shown to yield better protection and more cross-reactive antibody response than corresponding egg-adapted viruses with as few as one or two mutations, it says. If these mutations are located near the site where HA binds to host cells, they can "dramatically alter" vaccine efficacy.
Problem may persist
The authors write that one particular mutation (at position 156 of site B of the H3 globular head) may have particularly contributed to reducing antibody recognition and neutralization of circulating viruses.
The WHO, in its February 2013 recommendations on flu vaccine strains for 2013-14, noted that the egg-adapted version of the Victoria H3N2 strain, when tested in ferrets, yielded a limited antibody response to circulating H3N2 viruses. So the agency recommended using another egg-adapted strain, called A/Texas/50/2012, to minimize changes from the Victoria reference strain.
Skowronski and colleagues say the egg-adapted Texas strain lacks the key mutation at position 156, but it has two of the others that were in the 2012-13 egg-adapted vaccine strain. They tested 122 circulating H3N2 viruses against the egg-adapted Texas strain and found that 90% of them generated a reduced antibody response, suggesting that use of the Texas strain may not solve the problem of low VE. Last month the WHO picked the Texas strain for the 2014-15 Northern Hemisphere flu vaccine.
A call for careful monitoring
The authors say their findings point to a need for careful monitoring not only of circulating flu strains but of the annual vaccine components. And in reporting the match between circulating strains and the vaccine, they write, researchers should specify which vaccine version they're using, whether it's the cell-passaged or egg-passaged WHO prototype or the egg-adapted reassortant. Until now, reports on vaccine/virus match and VE have focused on the comparison between circulating viruses and the WHO reference strain.
"We need to be explicit about what we're talking about when we say the viruses are antigenically similar," Skowronski said.
Calling her team's findings novel, she said, "Recently people monitoring VE have been saying there doesn't appear to be any correlation between antigenic match and VE. Well, maybe that's because we've been comparing [circulating viruses] to the wrong reference strain."
She commented that monitoring the vaccine viruses could be helpful for public health messaging. "If the vaccine virus is not antigenically equivalent to what the WHO recommended, there's kind of a cap on how much protection it can provide right out the gate. If public health authorities know that, they might do some additional messaging, such as adjunctive measures."
Skowronski called for further research to try to pinpoint which mutations are most likely to affect antigenicity and VE and to see if they can be controlled.
"If we cannot control those mutations, then maybe we should be advocating for alternative approaches to egg-based production," she said. Cell-culture production "may be one approach to address, but there may be other approaches."
Only one cell-based flu vaccine, Novartis's Flucelvax, is currently licensed in the United States.
Only an association?
Other experts observe that it's been known for decades that mutations can occur along the pathway from a recommended reference virus to a reassortant that replicates quickly in eggs. They differ somewhat regarding the novelty of the Canadian findings.
Nancy Cox, PhD, director of the CDC's Influenza Division, said it's been known since the 1980s that HA mutations can crop up when flu viruses grown in mammalian cells are adapted to eggs. The phenomenon was first seen in influenza B viruses.
In the 1990s the CDC found that human flu viruses grown in animal cells were very similar to the clinical isolates they were derived from, but not always identical, she said. "The egg isolates usually had changes in the hemagglutinin," she added.
"When viruses taken from humans . . . are put in an avian substrate to grow, they need to change in order to grow well," she said. "So this is just a natural process that occurs. This is something that we have to deal with each and every year in the vaccine enterprise."
Proving a direct link between the mutations in the egg-adapted vaccine and reduced VE would require directly comparing the effectiveness of the egg-adapted version and reference strain it was based on, Cox said.
"Danuta [Skowronski] of course is looking at this from an epidemiologic standpoint, so she's found an association, which is what epidemiologists look for," she said, adding that it may or not be a causal association.
In her view, the findings are not necessarily new. "I think if you read the literature, you'll see plenty in there already, but it [the study] is reemphasizing in a modern context what has been known for three decades," she said.
She also observed that the WHO's flu vaccine consultants very carefully compare cell-grown and egg-grown versions of candidate viruses when recommending strains for the vaccine. More than 40 such pairs were examined at last month's meeting of the group in Geneva.
An 'important, solid study'
Richard Webby, PhD, a leading flu researcher who participates in the WHO's deliberations on which strains to include in the seasonal vaccine, agreed that the study shows an association only, but he welcomed the findings.
"It's an important, solid study," he said in an interview. "The important thing is it's an association, but it's an association that fundamentally makes sense." He is director of the WHO's collaborating influenza center at St. Jude Children's Research Hospital in Memphis.
Webby also commented that recent H3N2 strains are "a problematic group of viruses. . . . They are difficult to grow in eggs, which is not unheard of for H3N2, but the real issue that sets them apart is the fact that when you do get them growing well in eggs, to do so they change in positions that also affect their antigenicity."
Concerning the egg-adapted H3N2 Texas strain, which will be in this year's Southern Hemisphere flu vaccine as well as the 2014-15 vaccine, Webby said, "The Texas strain is improved. It's still not perfect, it still has some of the same problems, but not as many as the Victoria."
He added that he will be interested in any flu VE studies that come out of the Southern Hemisphere this year.
Another expert, Michael T. Osterholm, PhD, MPH, also praised the study: "This is the first time I'm aware of that low vaccine effectiveness has been tied to mutations in the egg-adapted vaccine strain. I think this very well could be a function of the fact that it's only in recent years that we've had the kind of VE data that could be matched up with this." It's "not a new phenomenon," but it has not been demonstrated before, he suggested.
Osterholm is director of the University of Minnesota's Center for Infectious Disease Research and Policy, publisher of CIDRAP News.
He agreed with Skowronski that the H3N2 Texas strain may limit VE against H3N2 next season. "The number of mutations is such that you could expect that next year we may well have inferior VE for H3N2," if H3N2 is a major player in the season, he said.
John Treanor, MD, a veteran flu vaccine researcher at the University of Rochester, commented that the potential for antigenic changes when flu viruses are egg-adapted, with possible effects on VE, have been a concern for a long time.
"This was first reported by James Robinson from the UK about 20 years ago, but it is not a consistent finding—some viruses really don't change much with egg adaptation, and some do," he commented.
Treanor added, "Last year, the CDC VE network reported extremely low effectiveness of the 2012-2013 vaccine against H3N2 viruses, especially in the elderly. I don't remember the exact details, but I believe one reason for this was felt to be differences arising from egg adaptation."
Cell-based alternative
Jeffery Duchin, MD, chief of the Communicable Disease Epidemiology and Prevention Section for Seattle and King County Public Health, called the Canadian study "a fascinating report with important implications for influenza vaccine production."
He added, "In my view, the study both suggests that it would be important to routinely monitor egg-adapted vaccine strains for antigenic changes relative to the recommended prototype virus for vaccine manufacturing and provides additional evidence of the need for alternative technologies to egg-based influenza vaccine production, such as cell-based production."
Unnamed experts at the WHO, in comments e-mailed by spokesman Glenn Thomas, said the Canadian findings are not new to the agency; they were presented and discussed at a WHO consultation meeting in September 2013.
The agency said the candidate vaccine viruses that it recommends are the best ones available for vaccine development and production.
"Further propagation of the A(H3N2) virus in eggs can lead to mutations occurring," the agency said. "It is one of the reasons for the argument for influenza viruses to be propagated in approved cell lines rather than eggs."
Webby said cell-based vaccine production could eliminate the problem of mutations due to egg adaptation. But he and others said there is a regulatory obstacle in that regard: Currently in the United States, all flu vaccine manufacturers must begin their process with an egg-adapted virus, even if they use cell culture production.
"At the present time the regulatory pathway requires that manufacturers begin with an egg isolated virus," said Cox. "There may be in the future a pathway that would involve isolation of flu viruses in certified cell lines, ones demonstrated to be free of adventitious agents and certified as valid for production of vaccine."
Treanor said he recalled that when the low VE was reported for H3N2 in 2012-13, "there was some controversy related to the requirement that even though Novartis was using a cell culture system [for Flucelvax], they were required to end up with a virus that antigenically matched the egg-grown virus."
Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014 Mar 25;9(3) [Full text]
See also:
February 2014 WHO technical report on flu vaccine strain selection for 2014-15
February 2013 WHO statement on strain selections for 2013-14 vaccine, with link to technical report
Feb 21, 2013, CIDRAP News story on midseason US flu vaccine effectiveness in 2012-13