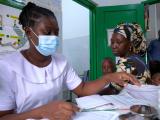
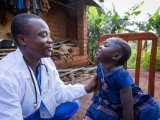
Novartis today announced that it has received approval for the world's first malaria treatment for newborns and young babies, paving the way for the drug to be used soon in eight African countries that participated in clinical trials.
The drug, called Coartem Baby (artemether-lumefantrine), was approved by Swiss drug regulators, and the eight African counties are expected to issue rapid approvals under the Swiss agency's marketing authorization for global health products process. Novartis developed the drug in collaboration with the Malaria for Medicines Venture (MMV), a Swiss-based nonprofit, along with other groups.
Treatment and vaccination gap for youngest babies
About 30 million babies are born in Africa's high-burden malaria countries every year, with 3.4% to 18.4% of infections in infants younger than 6 months old. However, there are no approved treatments for babies who weigh less than 4.5 kilograms (9.9 pounds), leaving a treatment gap. Also, there are no malaria vaccines approved for the youngest infants.
Vas Narasimhan, MD, chief executive officer of Novartis, said in the statement that Novartis has been pushing forward with malaria treatments for more than 30 years, with an eye toward creating breakthroughs where treatments are needed most. "Together with our partners, we are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve."
The company said it plans to introduce the treatment—dissolvable even in breast milk and made in a sweet cherry flavor—on a largely not-for-profit basis to increase access in malaria-endemic countries.
We are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve.
Umberto D'Alessandro, MD, PhD, who directs the Medical Research Council Unit, The Gambia (MRCG) at the London School of Hygiene and Tropical Medicine, said the youngest infants have typically been excluded from clinical trials. Newborns and young infants have immature liver function and may metabolize malaria drugs differently, which may mean the doses for older children may not be appropriate for small babies because of an increased risk of overdose and toxicity.
Swiss regulators based their approval on a phase 2/3 trial that examined a new ratio and dose of the drug, which is indicated for babies that weigh from 2 to 5 kg (4.4 to 11 pounds).