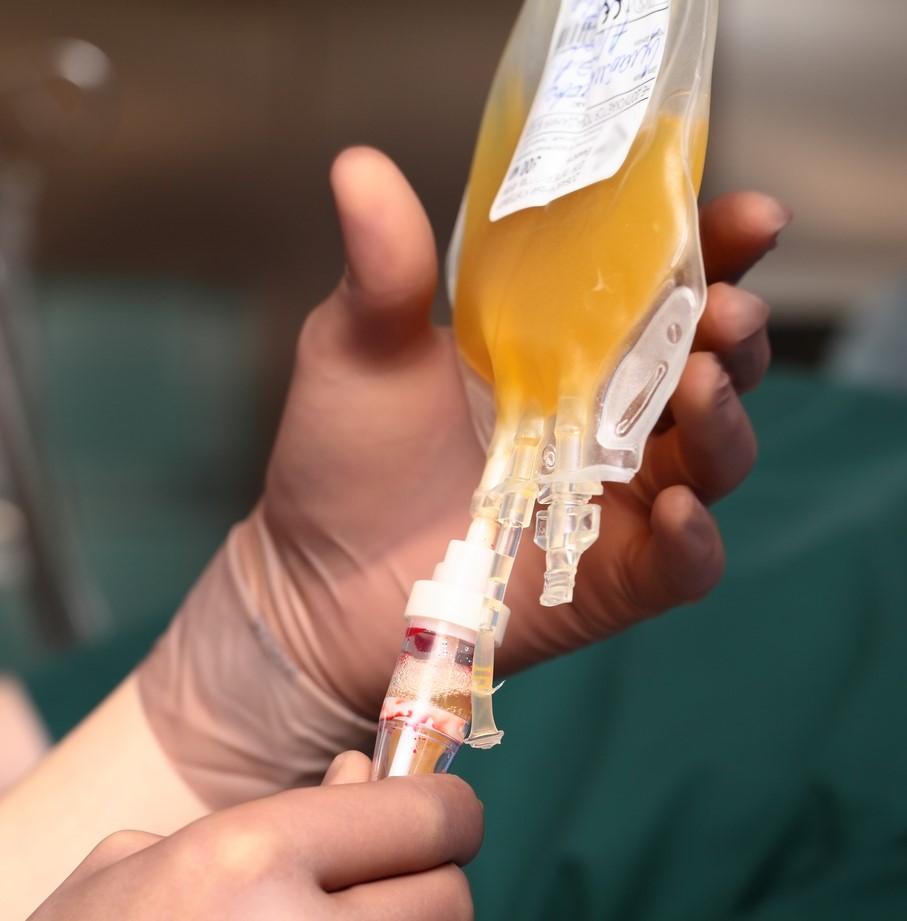
Yesterday the Food and Drug Administration (FDA) announced it has approved plasma from recovered COVID-19 patients to be used as a hospital-based treatment for the novel coronavirus under an emergency use authorization (EUA).
The move was announced during an unscheduled press conference by President Donald Trump yesterday evening, just a day before the Republican National Convention begins. Critics said Trump portrayed the EUA as a treatment breakthrough to boost support as the Nov 3 presidential election draws nearer.
"Based on the science and the data, the FDA has made the independent determination that the treatment is safe and very effective," Trump said. He also said, citing a non–peer-reviewed Mayo Clinic study, that the treatment reduced mortality in hospitalized patients by 35%.
Stephen Hahn, MD, the head of the FDA appeared alongside Trump and also spoke of the 35% reduction in mortality.
"A 35% improvement in survival is a pretty substantial clinical benefit. What that means is—and if the data continue to pan out—[of] 100 people who are sick with COVID-19, 35 would have been saved because of the admission of plasma," Hahn said.
But in a press release from the FDA last night Hahn was more circumspect.
"We're encouraged by the early promising data that we've seen about convalescent plasma. The data from studies conducted this year shows that plasma from patients who’ve recovered from COVID-19 has the potential to help treat those who are suffering from the effects of getting this terrible virus," said Hahn. "At the same time, we will continue to work with researchers to continue randomized clinical trials to study the safety and effectiveness of convalescent plasma in treating patients infected with the novel coronavirus."
Pressure to approve?
Hahn's press conference enthusiasm, according to the Washington Post, could be a sign he was buckling to political pressure from Trump, who tweeted over the weekend that the "deep state" was at work at the FDA, slowing down vaccine and treatment developments until after the presidential election.
Hahn's interpretation of the 35% reduction in mortality was widely disputed by scientists on Twitter, who used the same data from the Mayo Clinic to show that 3.2 people out of 100—not 35—would be saved by the administration of convalescent plasma. The difference is in relative versus absolute risk, with 35% being the percent improvement but, because the rate of those seeing improvement was so low—8.7% to 11.9%—the absolute reduction is much smaller.
Also, even for that small absolute improvement, the plasma would need to be administered within 3 days versus 7, according to the Mayo data.
Thomas File, MD, the president of the Infectious Disease Society of America (IDSA) also issued a cautious statement on the FDA's announcement.
"While the data to date show some positive signals that convalescent plasma can be helpful in treating individuals with COVID-19, especially if given early in the trajectory of disease, we lack the randomized controlled trial data we need to better understand its utility in COVID-19 treatment," File said.
"For this reason, IDSA supports the continued collection of data in randomized clinical trials to better understand the benefits of convalescent plasma treatment before authorizing its wider use in patients with COVID-19."
Case slowdown may be tied to interventions
US officials reported 34,567 new COVID-19 cases yesterday and 449 new deaths, according to the Johns Hopkins COVID-19 tracker, bringing the total to 5,730,294 cases and 177,065 fatalities.
Though the country still has by far the most cases in the world, the daily case counts have decreased in the past month, and experts say the decline is linked to interventions, including statewide mask mandates and the shuttering of indoor bars and restaurants, gyms, and theaters.
Even after a summer of surging case counts, the United States remains deeply politically divided over the pandemic. A new poll conducted by CBS News/YouGov shows that 73% of Republican voters compared with 38% of all voters say the country is dealing with the pandemic well. Sixty-four percent of Republicans think deaths from COVID-19 are fewer than those reported—compared with 36% of all voters—and 57% of Republicans vs 10% of Democrats said the number of US deaths due to the virus is acceptable.
Other US developments
- Texas and Louisiana are bracing for two tropical storms, Marco and Laura, this week. Officials said they are worried the storms could compound efforts to control COVID-19 in those states.
- The Centers for Disease Control and Prevention no longer recommends that travelers from outside the US self-quarantine for 14 days upon entering this country.