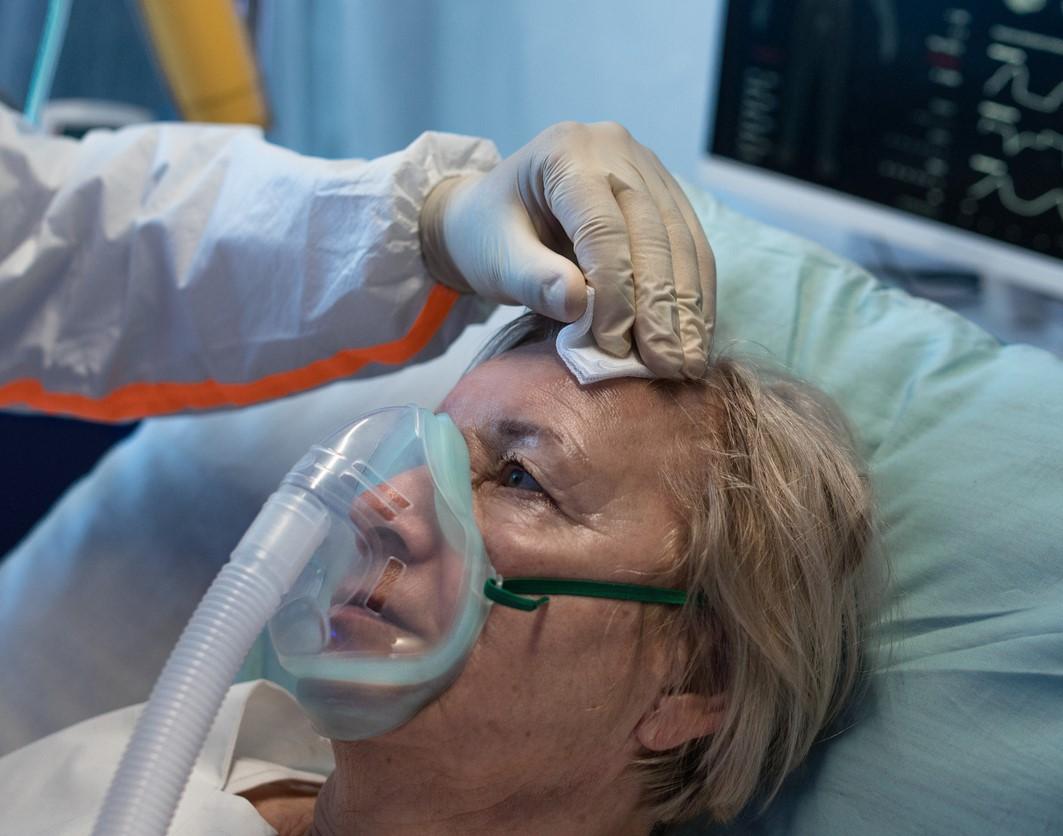
Hospitalized COVID-19 patients given the anti-inflammatory monoclonal antibody tocilizumab were less likely to die or require invasive mechanical ventilation, according to preliminary results of the UK RECOVERY trial posted today on the medRxiv preprint server.
Led by University of Oxford researchers, the ongoing Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial involved assigning 4,116 severely ill coronavirus patients to receive either intravenous tocilizumab, a rheumatoid arthritis drug, or usual care. Most (82%) of the participants also received a systemic corticosteroid such as dexamethasone.
Higher odds of leaving the hospital alive
Of the 2,022 patients receiving tocilizumab from Apr 23, 2020, to Jan 25, 2021, 596 (29%) died within 28 days of hospitalization, versus 694 of 2,094 (33%) of patients receiving usual care (rate ratio [RR], 0.86), for a 4% absolute difference. The drug also raised the chances of being released from the hospital alive within 28 days from 47% to 54% (RR, 1.22).
Patients in the tocilizumab group not receiving invasive mechanical ventilation when enrolled in the trial also were also less likely to advance to needing that treatment (from 38% to 33%; RR, 0.85), and fewer went on to need dialysis (5% vs 7%).
But the drug didn't affect rates of successful weaning from mechanical ventilation or the subsequent need for noninvasive or invasive respiratory support among those who required no such support at study enrollment. The authors said that the benefits of tocilizumab seemed particularly apparent in those also receiving a systemic corticosteroid but added that it may have been due to chance.
All patient subgroups saw benefits, including those who needed supplemental oxygen with a face mask and those needing mechanical ventilation in an intensive care unit. Mean participant age was 63.6 years.
Among the 4,116 participants, 562 (14%) received invasive mechanical ventilation, 1,686 (41%) received noninvasive respiratory support, and 1,868 (45%) received no respiratory support except supplemental oxygen. All patient had low oxygen levels and signs of inflammation.
Three serious adverse effects were attributed to tocilizumab, including ear infection, Staphylococcus aureus bloodstream infection, and lung abscess. All conditions resolved after treatment.
One-third to one-half fewer deaths
RECOVERY researchers have been testing different possible treatments for coronavirus since March 2020, with tocilizumab added to the trial in April. As recently as Feb 3, the US National Institutes of Health said that the data to recommend for or against the use of tocilizumab in patients requiring invasive or noninvasive mechanical ventilation or oxygen are insufficient, but the researchers said that their findings require an update to those guidelines, as well as action to increase tocilizumab's global availability and affordability.
"Our data suggest that in COVID-19 patients who are hypoxic and have evidence of systematic inflammation, treatment with a combination of a systemic corticosteroid plus tocilizumab would be expected to reduce mortality by about one-third for patients receiving simple oxygen and nearly one-half for those receiving invasive mechanical ventilation," the researchers wrote.
The authors noted that seven previous randomized studies of tocilizumab in COVID-19 patients have produced mixed results that, taken together, didn't suggest a significant reduction in death rates.
They said, however, that the RECOVERY trial has more than three times as many deaths to study than in the previous trials combined. Thus, they added, all eight trials together showed that the drug is linked to a 13% drop in death by 28 days (RR, 0.87).
Lead author Peter Horby, PhD, of the University of Oxford, called the findings of the benefits of tocilizumab plus dexamethasone "impressive and very welcome" in a news article on the RECOVERY website. "Previous trials of tocilizumab had shown mixed results, and it was unclear which patients might benefit from the treatment," he said. "We now know that the benefits of tocilizumab extend to all COVID patients with low oxygen levels and significant inflammation."
The authors say the results will be submitted to a peer-reviewed medical journal.