The US Food and Drug Administration (FDA) has created a new website that could help clinicians make more informed decisions about antimicrobial use and prevent the spread of drug resistance, the agency announced today in a press release.
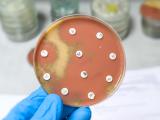
The website will allow the agency to more quickly update antimicrobial susceptibility test interpretive criteria—commonly known as "breakpoints"—for antibiotic and antifungal drugs. Antimicrobial susceptibility tests rely on these breakpoints to determine whether specific bacteria or fungi that are causing a patient's infection are susceptible or resistant to certain drugs. Clinicians use susceptibility test results to guide therapy decisions.
Previously, drug manufacturers had to update drug labeling with new breakpoints, and updated labels had to be reviewed and approved by the FDA on a case-by-case basis. Only then could the companies who manufacture corresponding antimicrobial susceptibility tests update testing criteria and device labeling. The agency says this process was inefficient and created an unnecessary delay in getting updated breakpoints to healthcare professionals.
With the new website, drug companies won't have to update their labeling every time the breakpoint for a certain drug changes. Instead, labeling will have to be updated only once, to direct providers to the FDA website containing updated breakpoint information. The FDA retains full authority to accept and recognize the updated breakpoints, or to establish alternative breakpoints.
Streamlining the process
"Prescribing a drug that's only going to be met with resistance from the bacteria or fungus it's intended to treat doesn't help that patient, and it has broader public health consequences that cannot be ignored," FDA Commissioner Scott Gottlieb, MD, said in the release.
"Under the old approach, it took too long to update each individual drug's labeling with information needed for susceptibility testing and it was clear [that] a more centralized approach was needed."
Gottlieb said the new website is aimed at making the process "more efficient and informed."
More timely updating of breakpoints can also help hospitals—which use susceptibility tests to monitor resistance levels—determine if additional infection control measures are needed to prevent resistant pathogens from spreading.
The agency was required to create the website under a provision of the 21st Century Cures Act that called for speeding up the recognition of updated antimicrobial breakpoints and getting that information to healthcare providers more quickly.
See also:
Dec 13 FDA press release
New FDA website