A non–peer-reviewed study published on the preprint server bioRxiv suggests that highly pathogenic avian influenza (HPAI) virus shed in poultry droppings can be transmitted by the wind, a possibility that other experts say can't be ruled out but is also very difficult to prove.
The report centers on a February 2024 outbreak of H5N1 avian flu among unrelated commercial poultry farms located about 8 kilometers (5 miles) apart in the Czech Republic during the 2023-24 HPAI season.
Since the latest H5N1 epidemic began in February 2022, the virus has led to the deaths of more than 150 million US birds.
Ideal weather for transmission
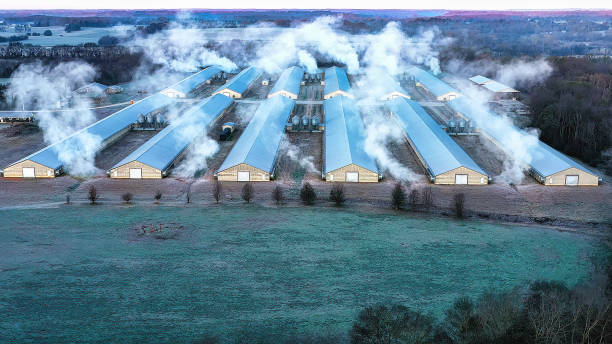
The cluster was identified after avian flu killed 5,000 fattening ducks within 2 days at a basic-biosecurity 50,000-bird farm near a lake frequented by wild ducks, the probable cause of the outbreak; the rest of the poultry were culled. A week later, deadly outbreaks with a slower disease course occurred at two high-biosecurity chicken farms, where dead birds were found mainly near the barns' air-intake vents; the entire population was culled. All farms had their own wells.
Experts hypothesize that the slower disease course in the chicken barns may be due to lower H5N1 transmissibility in chickens, waning concentrations of the virus over distance from the duck farm, or housing conditions.
The researchers, from the State Veterinary Institute Prague, used genetic, epizootiologic, meteorologic, and geographic data to reconstruct events suggesting that wind was the mechanism of H5N1 transmission between poultry on at least two of the farms. Three H5N1 strains collected from birds at all three farms were genetically identical, no large waterways were near the chicken farms, and the duck farm didn't share staff or contractors with the chicken farms.
Our results suggest that the contaminated plume emitted from the infected fattening duck farm was the critical medium of HPAI transmission, rather than the dust generated during depopulation.
What's more, meteorologic data revealed a breeze in the direction of the chicken farms, cloudy conditions that could have kept the sun's ultraviolet light from killing the virus, and virus-supporting cool air. The team didn't conduct air sampling.
"Our results suggest that the contaminated plume emitted from the infected fattening duck farm was the critical medium of HPAI transmission, rather than the dust generated during depopulation," the study authors wrote. "They also strongly implicate the role of confined mechanically-ventilated buildings with high population densities in facilitating windborne transmission and propagating virus concentrations below the minimum infectious dose at the recipient sites."
Airborne not only or major mode of spread
Lead study author Alexander Nagy, PhD, told CIDRAP News that he and his team became convinced about the role of windborne transmission in these outbreaks when they confirmed the identical identity of the H5N1 strain in the donor and recipient farms, the nearly ideal weather conditions during the transmission event, and the slow disease progression in the chicken barns characterized by feed and water consumption before clinical signs appeared.
"Additionally, the affected birds [dead chickens] were located in sections closest to the air inlets," he said. "Finally, we excluded all other plausible explanations."

Michael Osterholm, PhD, MPH, director of the University of Minnesota's Center for Infectious Disease Research and Policy (CIDRAP), publisher of CIDRAP News, said airborne transmission can be very important, "meaning that that's the most logical explanation for when you have many barns with outbreaks in one geographic area where human biosecurity cannot be implicated as a reason for transmission."
In the past, he said, the poultry industry has been reluctant to acknowledge airborne transmission because of the implications it may have for its practices: "The industry's reluctance to accept this possibility is not that dissimilar to what we saw with the lack of some in the medical and public health communities to recognize that SARS-CoV-2 transmission was also airborne."
While the researchers did a very good job of laying out their hypothesis and supporting data, their conclusion should be interpreted with caution, said David Swayne, DVM, PhD, a poultry veterinarian who retired as an avian flu researcher with the US Department of Agriculture (USDA) Agriculture Research Service.
"I think we, as veterinarians who deal with avian influenza and other infectious diseases, would acknowledge that there is some airborne—and I'll use the word dissemination—and that may lead to transmission," he said. "But we have to be cautious to make sure people understand that it doesn't mean that it's the only way, nor that it's the major way. And each individual facility is going to be different."
Montserrat Torremorell, DVM, PhD, chair of the Department of Veterinary Population Medicine at the University of Minnesota, called the researchers' argument for airborne transmission "compelling."
"Meteorological conditions, timing of infection, housing conditions of the animals, susceptibility of the animal populations that became infected and the lack of other epidemiological links between the premises are supportive of airborne transmission in this case," she said in an email.
During an avian flu outbreak in Minnesota, Torremorell collected air samples inside and outside facilities housing three infected turkey and three egg-laying chicken flocks. Air samples from five of six flocks tested positive for large quantities of H5N1 virus, all of them in the active infection stage. The negative sample was from a flock in the advanced stage of depopulation.
"The larger number of positive samples were inside the facility and at the exhaust fan (~5 m [meters; 16 feet] away from the facility), and the number of positives decreased with distance, but even with that we identified some suspects (traces of RNA material) at about 150 m and 1 km [kilometer; roughly a half mile)," she said. "Viable virus (through virus isolation) was found inside the facilities, at the outside of the exhaust fan and at about 100 m."
Entry mechanism difficult to determine
David Stallknecht, PhD, professor emeritus at the University of Georgia's College of Veterinary Medicine and a wildlife expert, said the study provides additional circumstantial evidence to several studies suggesting windborne viral spread. But he added that the mechanism of disease transmission into a poultry house is hardly ever identified, because there is no way to control for variables.
"It basically says that it could have happened, and I would not dispute that," he said. "But to actually come down with concrete proof like you would in an experimental controlled experiment, there's too much going on."
"Influenza can be transmitted by a million different ways, probably many of them we don't even know about," he added. For example, whether the virus entered the poultry house via a raccoon, bird, person, or a person's shoes, "those kind of details never really get resolved."

Stallknecht said he and his team tried to research water-based avian flu transmission associated with ducks. "And we got to the point where we just had to quit because the numbers and the probabilities were so extreme," he said. "You can't do an experiment to pick up a one-in-a-trillion event."
Swayne said that moving equipment and people in and out of poultry barns has to be done at a very high level of biosecurity to prevent viral transmission: "Yes, you could have movement of the virus by the wind, but it may be that it's tracked in to the barns by the contamination around the outside, either from wild birds depositing fecal material there as they eat, spilled feed off of grain silos, etc."
He added that just because a virus is transported via the wind doesn't necessarily mean it will enter a barn that way. "The virus can move on particles or water droplets by the wind for a distance, and then it could land and contaminate the environment."
Authors were initially skeptical, too
Nagy acknowledged that airborne spread isn't typically considered a primary route of infection in poultry, and windborne spread requires "a constellation of many specific conditions" that behooves further research.
"However, when all conditions align, windborne transmission becomes a feasible mode of spread and could play a more significant role than previously thought, especially in densely populated poultry areas," he said. "The windborne route is important to consider in cases when the infection emerges in new locations without obvious links to other farms or when illness and mortality are first observed near the air inlets."
Poultry operations typically consist of confined, mechanically ventilated buildings with high population densities, a situation unlikely to change.