The US Department of Health and Human Services (HHS) will likely remove recommendations from the Centers for Disease Control and Prevention (CDC) that children, teens, and pregnant women receive the COVID-19 vaccine, the Wall Street Journal (WSJ) reported yesterday, citing people familiar with the developments.
The CDC currently recommends that everyone ages 6 months and older be vaccinated against COVID, a universal recommendation that it has had in place since September 2023, when the CDC's Advisory Committee on Immunization Practices (ACIP) voted for that option.
At the time, some ACIP members thought it would be better to focus on seniors and those at highest risk for disease, but the wider committee was swayed by data, including those for long COVID, showing that no group is at low risk for the virus. The universal COVID vaccine recommendation is similar to the that for influenza vaccines, and health officials have said another benefit of the approach is simplified vaccine messaging.
Sources also told the WSJ that the HHS recommendation change will occur when the Food and Drug Administration launches a new framework for approving vaccines, which could come within the coming days.
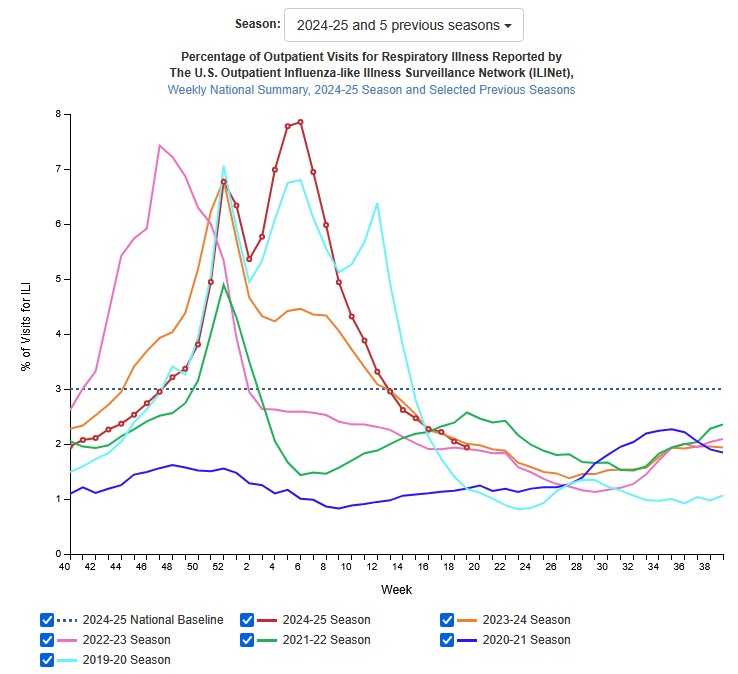 US flu activity is low and declining further, according to the latest
US flu activity is low and declining further, according to the latest 












