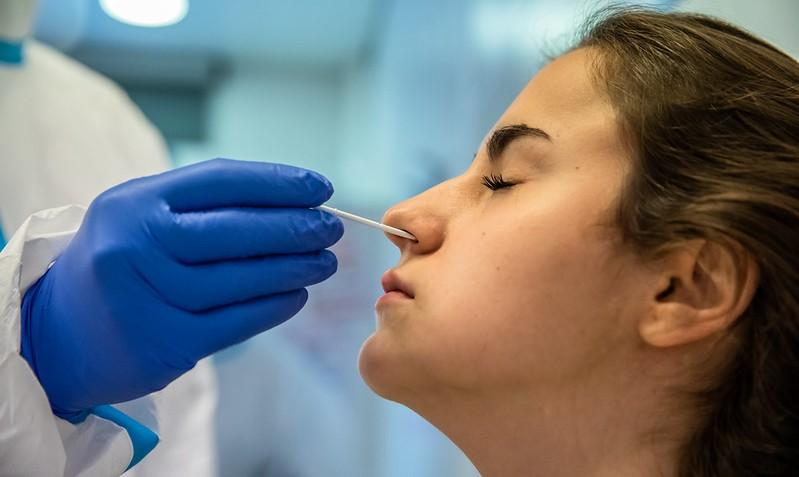
Today in JAMA Network Open, a randomized clinical trial shows that a single healthcare worker (HCW)-collected throat swab had significantly higher sensitivity for COVID-19 rapid antigen testing (RAT) than an HCW-collected nose swab during Omicron predominance, but self-collected nose swabs were more sensitive than self-collected throat swabs among participants with symptoms.
For the trial, a team led by Copenhagen University Hospital researchers in Denmark randomly assigned 2,674 people aged 16 and older being tested for COVID-19 by reverse transcription-polymerase chain reaction (RT-PCR) to self- or HCW-collected throat and nasal swabs for RAT in February and March 2022.
Four samples (two HCW-collected nose and throat swabs for RT-PCR and two self- or HCW-collected swabs for RAT) were collected per participant at two urban COVID-19 outpatient test centers in Copenhagen, and additional HCW-collected throat and nose swabs were used as the reference standard.
The median participant age was 40 years, 57.4% were women, 40.2% had symptoms, and 30.9% tested positive for COVID-19 on RT-PCR from throat (4.2%) or nasal (1.8%) specimens or both (25.0%).
Throat specimens may be key to infection
The researchers said that RATs have been widely used because they are inexpensive and available over the counter for home use, allowing early detection and isolation of infectious people. "However, the rate of false-negative home-based rapid antigen test results has been widely debated during the Omicron variant surge, and it has been suggested that throat swab specimens could improve test sensitivity," they wrote.
The authors noted that while health officials in Canada and the United Kingdom recommend using both nasal and throat specimens for RT-PCR, the US Food and Drug Administration authorized RAT for use with nasal specimens only.
"Research with molecular SARS-CoV-2 testing suggests that throat specimens play an important role in the initial infection, while studies with rapid antigen tests are contradictive and range from advising against to recommending the use of throat specimens," they wrote.
Self-throat sampling more challenging
The SARS-CoV-2 detection rate was 86.6% (95% CI, 84.3% to 88.9%) for a nose swab and 94.2% (95% CI, 92.6% to 95.8%) for a throat specimen. The median Ct value (viral load) was lower for HCW-collected nasal swabs than for HCW-collected throat swabs (16.7 vs 22.8). Of 286 samples sequenced for variants, most were either Omicron BA.2 (66.8%) or BA.2.9 (26.2%).
HCW-collected throat swabs had higher average sensitivity than HCW-collected nasal specimens for RAT (69.4% [95% confidence interval (CI), 65.1% to 73.6%] vs 60.0% [95% CI, 55.4% to 64.5%]). But in a subgroup analysis of symptomatic participants, self-collected nose swabs were more sensitive than self-collected throat swabs for RAT (mean sensitivity, 71.5% [95% CI, 65.3% to 77.6%] vs 58.0% [95% CI, 51.2% to 64.7%]).
Our findings suggest that the current testing recommendations should include throat specimens to improve the sensitivity of rapid antigen testing.
The findings "demonstrate that the throat sample technique is more challenging than obtaining a nasal sample, as we found a lower sensitivity and a higher number of inconclusive rapid antigen test results for self-collected throat specimens compared with HCW-collected throat specimens," the researchers wrote. "In contrast, no difference was found between HCW-collected and self-collected nasal specimens."
Using both nasal and throat swabs increased sensitivity for HCW- and self-collected specimens by 21.4 and 15.5 percentage points, respectively, compared with a single nose swab.
"Our findings suggest that the current testing recommendations should include throat specimens to improve the sensitivity of rapid antigen testing," the study authors wrote. "Further research should confirm our findings using redesigned and other rapid antigen testing devices and explore whether throat specimens can also improve the detection of other common airway infections."