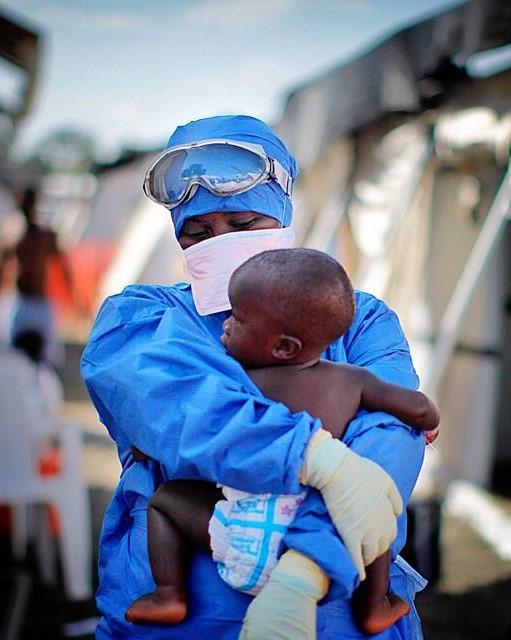
Over the Thanksgiving holiday period and through today, the Democratic Republic of the Congo (DRC) reported 33 more Ebola cases, vaulting the total past 400, as the country's health officials announced the launch of the first clinical trial of experimental drugs to treat the disease.
Meanwhile, in its latest weekly update on the outbreak, the World Health Organization (WHO) said transmission continues in several North Kivu province cities and villages. Currently, the three main hot spots are Kalunguta, located in a security "red zone," Beni, and the Butembo/Katwa area.
The WHO said the Ebola situation in the DRC remains complex and challenging, but it is still confident that the DRC and its partners can successfully contain the outbreak.
Health center exposures, infections in babies
In its update, the WHO said health centers have been identified as a source of disease transmission and that medications given as injections are a notable cause of disease spread. The agency added that efforts are under way to improve infection prevention and control, provide water and products for cleaning, training health providers, and encouraging treatment with medications that don't require injections.
Spread of the virus in health facilities that aren't Ebola treatment centers has occurred in earlier outbreaks, and infections among healthcare workers are known to amplify outbreaks. A recent report noted a spurt of new infections in healthcare workers, and the WHO's latest reports say 39 have been infected so far, at least 10 of them fatally.
Of 36 cases confirmed from Nov 14 to Nov 20, 7 involved newborn babies and 6 were in children ages 2 to 17. One of the illnesses was in a pregnant woman. The high numbers of illnesses in children and in women has been an unusual feature of the DRC's outbreak. According to the latest numbers, 61% of confirmed and probable case-patients have been female.
33 new cases, 21 more deaths
In daily updates spanning Nov 22 through Nov 25, the DRC's health ministry reported 33 more Ebola cases, lifting the total to 419 illnesses, including 372 confirmed and 47 probable cases.
The latest cases were reported in Beni (12), Katwa (9), Kalunguta (5), Butembo (6), and Oicha (1). At this rate, the epidemic will soon surpass the world's second-largest Ebola outbreak ever, which occurred in Uganda in 2000 and involved 425 cases.
In its update today, the health ministry said officials are investigating 59 suspected Ebola cases. Since Nov 21, the ministry reported 21 more deaths, raising the outbreak's fatality count to 240.
In its Nov 23 update, the health ministry said that, because of an increase in cases in Butembo and surrounding areas, outbreak coordinators decided to send additional response teams to the areas of Katwa, Kaluguta, and Lubero. The team sent to Lubero will be tasked with strengthening epidemiologic surveillance and training health workers on how to identify and treat suspected cases and high-risk contacts from Butembo.
The ministry said today that 35,958 people have now been vaccinated. At least 10,110 are frontline health workers.
Treatment trial launch
In another new outbreak development, the health ministry said in a Nov 24 announcement that an ethics committee at the University of Kinshasa has approved a protocol for testing four experimental Ebola treatments, which are currently being used on an emergency basis in Ebola treatment centers. They are mAb114, Zmapp, remdesivir, and Regeneron's REGN-EB3 antibody.
The authorization for emergency or compassionate use, however, doesn't provide for the standardized collection of data on the efficacy and safety of each treatment the health ministry said.
The ministry statement indicates that the clinical trial started last week on three of the four treatments (Zmapp, mAb114, and remdesivir) at Beni's Ebola treatment center. Future trials could be extended to other sites and include REGN-EB3. However, for now, the other Ebola treatment centers will continue to administer the drugs on a compassionate basis.
As with compassionate use treatment, patients or families will need to give informed consent to participate in clinical trials, but the main difference will be that the choice of treatment will be randomized by lottery rather than selected by doctors.
The number of patients in the trial will depend on how the outbreak evolves and how willing patients are to participate, the ministry said. It added that data in the North Kivu outbreak alone aren't likely to be enough to draw firm conclusions about the treatments, and the clinical trials may extend over 5 years and cover several outbreaks in multiple affected countries.
160 have received experimental treatment
WHO Director-General Tedros Adhanom Ghebreyesus, PhD, said in a WHO statement today, "While our focus remains on bringing this outbreak to an end, the launch of the randomized control trial in DRC is an important step towards finally finding an Ebola treatment that will save lives."
The WHO said 160 patients in the DRC have already been treated with experimental drugs as part of the emergency use protocol.
The randomized clinical trial protocol is part of a multi-outbreak, multi-country study agreed to in a meeting of experts, partners, and other stakeholders convened by the WHO in October. That meeting resulted in a research and development blueprint for Ebola treatment clinical trials, which is published in a 14-page document on the WHO's website.
In its statement, the WHO said it is coordinating the trial, which is led and sponsored by the DRC's National Institute for Biomedical Research with its partners at the country's health ministry, the US National Institute for Allergy and Infectious Diseases, the Alliance for International Medical Action, and other groups.
See also:
Nov 22 DRC update
Nov 23 DRC update
Nov 24 DRC update
Nov 25 DRC update
Nov 26 DRC update
Nov 21 WHO situation update
Nov 22 WHO statement
Nov 24 DRC clinical trial announcement
Nov 26 WHO clinical trial announcement
Oct 11 WHO Ebola therapeutics R&D blueprint