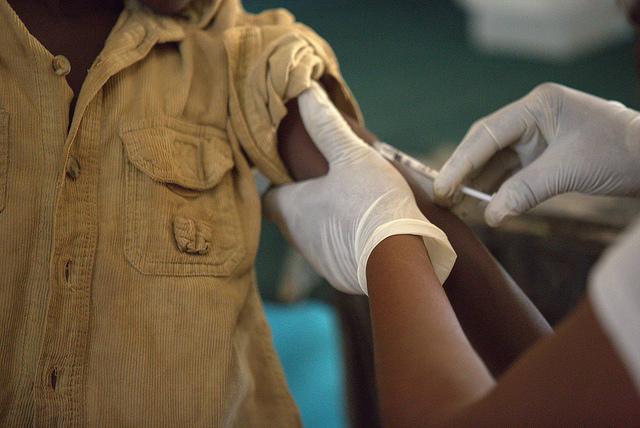
The World Health Organization (WHO) announced today that a phase 3 trial of a Canadian-developed Ebola vaccine will launch this week in Guinea, with a ring vaccination strategy designed to test if vaccines might be useful for stamping out hot spots in outbreak settings.
In a separate development involving the same vaccine, a research team from the United States reported their experience using it to try to prevent Ebola infection in a doctor who sustained a needlestick injury while treating patients in Sierra Leone. The doctor tested negative for Ebola, but the effect of the vaccine was unclear, the report said.
The vaccine, called VSV-EBOV, uses an Ebola virus protein spliced into a vesicular stomatitis virus (VSV). The vaccine was developed by researchers from the Public Health Agency of Canada (PHAC) and has been licensed to NewLink Genetics and Merck. A phase 3 clinical trial involving it and another vaccine candidate, using a different trial design, is already underway in Liberia.
Trial targets hot spot
Guinea's trial, set to launch on Mar 7, will be conducted by experts from the WHO, Guinea's health ministry, Doctors without Borders (MSF), and Norway. The Canadian government has trained and supported African research teams involved in the trial and is providing technical advice, the WHO said in its statement today.
The trial will take place in a region of Guinea that has the nation's highest number of cases. The ring vaccination strategy has been used in the past to eradicate smallpox, and the international team that put together the trial design includes Donald A. Henderson, MD, who spearheaded the WHO's smallpox eradication effort.
Marie-Paule Kieny, PhD, WHO assistant director-general and head of its Ebola research and development team, said in the statement that though Ebola cases are declining, the world can't let down its guard until the region reaches zero cases. "An effective vaccine to control current flare-ups could be the game-changer to finally end this epidemic and an insurance policy for any future ones," she said.
Once a new Ebola case-patient is diagnosed, researchers will identify contacts and offer them the vaccine. The goal of the trial is to assess if the vaccine protects people who were potentially exposed to the virus and if vaccinating contacts could create a buffer of protection to curb the spread of the virus. The WHO said frontline health workers will also be offered the vaccine.
The WHO's Ebola intervention advisory group selected VSV-EBOV for the Guinea trial because it met certain parameters, which included an acceptable safety profile, an appropriate immune response that included neutralizing antibodies, and timely availability of enough doses. The WHO said a second vaccine will be tested in a sequential study when supply is available.
Case report details postexposure use
Meanwhile, the report on the use of VSV-EBOV as prophylactic treatment after a needlestick injury in Sierra Leone appeared today in the Journal of the American Medical Association (JAMA). The injured doctor received a dose of the vaccine, flown to Sierra Leone on an emergency basis, 43 hours after the late-September injury. He declined other interventions besides the vaccine, and it was administered as he was medically evacuated to the United States.
The 44-year-old doctor, who was previously identified as Lewis Rubinson, MD, PhD, was observed and cared for at the National Institutes of Health Clinical Center in Bethesda, Md. He directs the critical care resuscitation unit at the University of Maryland's Shock Trauma Center.
VSV-EBOV has shown promise as a postexposure treatment in animal studies, and it has been used only once before for that purpose in a human—in 2009 for a lab worker in Germany who also suffered a needlestick injury.
After he received the vaccine, Rubinson had fever, nausea, and malaise, which progressively resolved over the next 3 to 5 days. Testing found no evidence of Ebola infection, and blood tests showed the vaccine provoked an IgG antibody response against the Ebola virus glycoprotein at a level linked to protection in animal studies.
Researchers concluded that it's not possible to gauge the safety and efficacy of VSV-EBOV for postexposure treatment from the case findings, but they said the clinical and lab findings could be helpful at a time when experts are gathering all the information they can about Ebola vaccines.
They added that the patient had a self-limited moderate-to-severe clinical syndrome that began 12 hours after receiving the vaccine, and future decisions on its postexposure use will need to balance the risk of harm with the risk of Ebola infection.
Difficult to weigh benefits, side effects
In a related editorial in the same issue of JAMA, Thomas Geisbert, PhD, an expert on hemorrhagic fever viruses with the University of Texas Medical Branch at Galveston, wrote that it's impossible to draw definitive conclusions from a single case report, and the negative tests for Ebola likely pointed to lack of an actual exposure. However, he said the findings can't rule out that the vaccine had an impact on viral replication.
The patient reported having traveler's diarrhea before the needlestick injury occurred, so it's difficult to assess the vaccine's safety profile, Geisbert added.
He said the need for antiviral treatment for Ebola is critical, but noted that the most effective tools for controlling outbreaks are vaccines and personal protective equipment.
Adverse events with VSV-EBOV wouldn't be unusual, because it uses a replication-competent virus, Geisbert said. He noted that Profectus Biosciences is developing a next-generation VSV-based Ebola vaccine with the goal of enhancing its safety, and that phase 1 trials are expected soon that may show if it would be safer than the first-generation vaccine.
Having a variety of companies working on Ebola vaccines should lead to an approved vaccine for human use in the near future, he wrote. Even if vaccination of large populations where the disease is endemic isn't practical, having a vaccine for healthcare providers and other responders will be useful, and ring-vaccination strategies might be useful in suppressing outbreaks, especially if a rapid-acting single-injection vaccine, such as aVSV-based vaccine, is available, Geisbert added.
Other developments
- Liberia's health ministry today said the country's last confirmed Ebola patient has been discharged, raising hopes that the country will soon be declared free of the disease, Agence France-Presse (AFP) reported. The patient left a Chinese-built Ebola treatment unit in the Monrovia suburb of Paynesville. Deputy Health Minister Tolbert Nyenswah said Liberia has gone 13 days without a new case. Yesterday the WHO said Liberia, for the first time during the outbreak, had no confirmed cases in the previous week. With Ebola still widespread in Sierra Leone and with case numbers constantly fluctuating in Guinea, health officials have warned that progress is fragile and that eliminating all cases throughout the region will be difficult.
- In an update today, the WHO said the number of confirmed, probable, and suspected Ebola infections in the outbreak region is at 23,983, which includes 9,823 deaths. The total reflect cases reported in Guinea and Sierra Leone as of Mar 3 and cases in Liberia as of Mar 1. Today's figures show an increase of 49 illnesses and 31 deaths compared to the numbers the WHO posted yesterday.
See also:
Mar 5 WHO statement
Mar 5 JAMA report
Mar 5 JAMA editorial
Mar 5 AFP story
Mar 5 WHO update