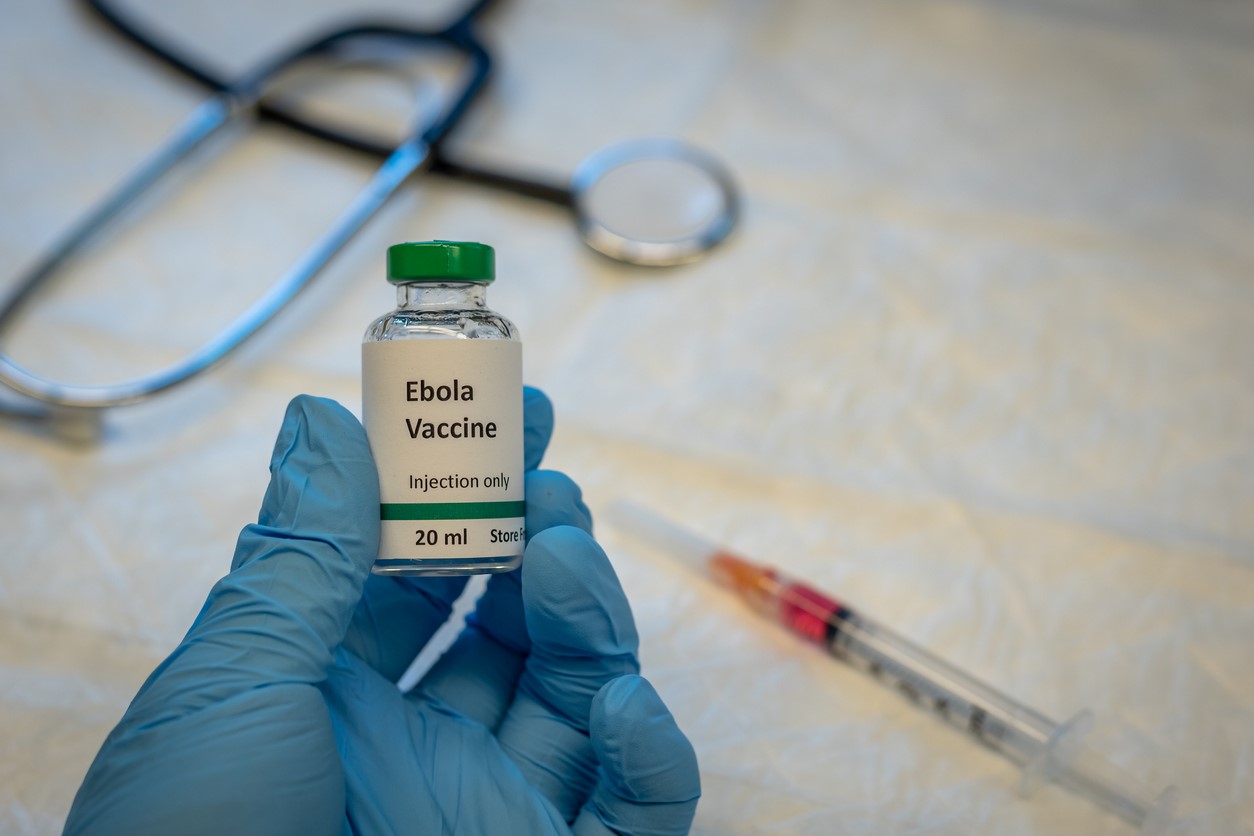
The International AIDS Vaccine Initiative (IAVI) recently announced the launch of the first human trial of a Sudan Ebola virus vaccine, an investigational vesicular stomatitis virus (VSV) vector vaccine donated by Merck and licensed by IAVI.
Currently, there are no approved vaccines or treatments for Sudan Ebola. An outbreak in Uganda last year resulted in 164 cases, 55 of them fatal. During the outbreak, a World Health Organization (WHO) working group recommended that ring vaccine trials with three potential candidate vaccines prioritize the VSV-EBOV vaccine licensed by IAVI, because it is made with the sample platform as the approved VSV-EBOV vaccine for the Zaire strain, which has shown to be safe and effective.
In a statement, IAVI said the first-in-human phase 1 trial is funded by the US Biomedical Advanced Research and Development Authority (BARDA), which is part of the Department of Health and Human Services (HHS). IAVI said it is the vaccine candidate's regulatory sponsor and is responsible for all future aspects of its clinical development, including how it measures up against IAVI's other Sudan Ebola vaccine, which uses the same virus vector but is made using a new production platform.
The trial will enroll 36 healthy adults at two clinical sites in the United States. The blinded placebo-controlled study will assess three dosage levels of the intramuscular shot, and researchers will track safety and immune response in the participants for 6 months after vaccination.
 A pilot study conducted at an academic medical center highlights the benefits of direct review of antibiotic prescriptions by pharmacists, researchers
A pilot study conducted at an academic medical center highlights the benefits of direct review of antibiotic prescriptions by pharmacists, researchers 









