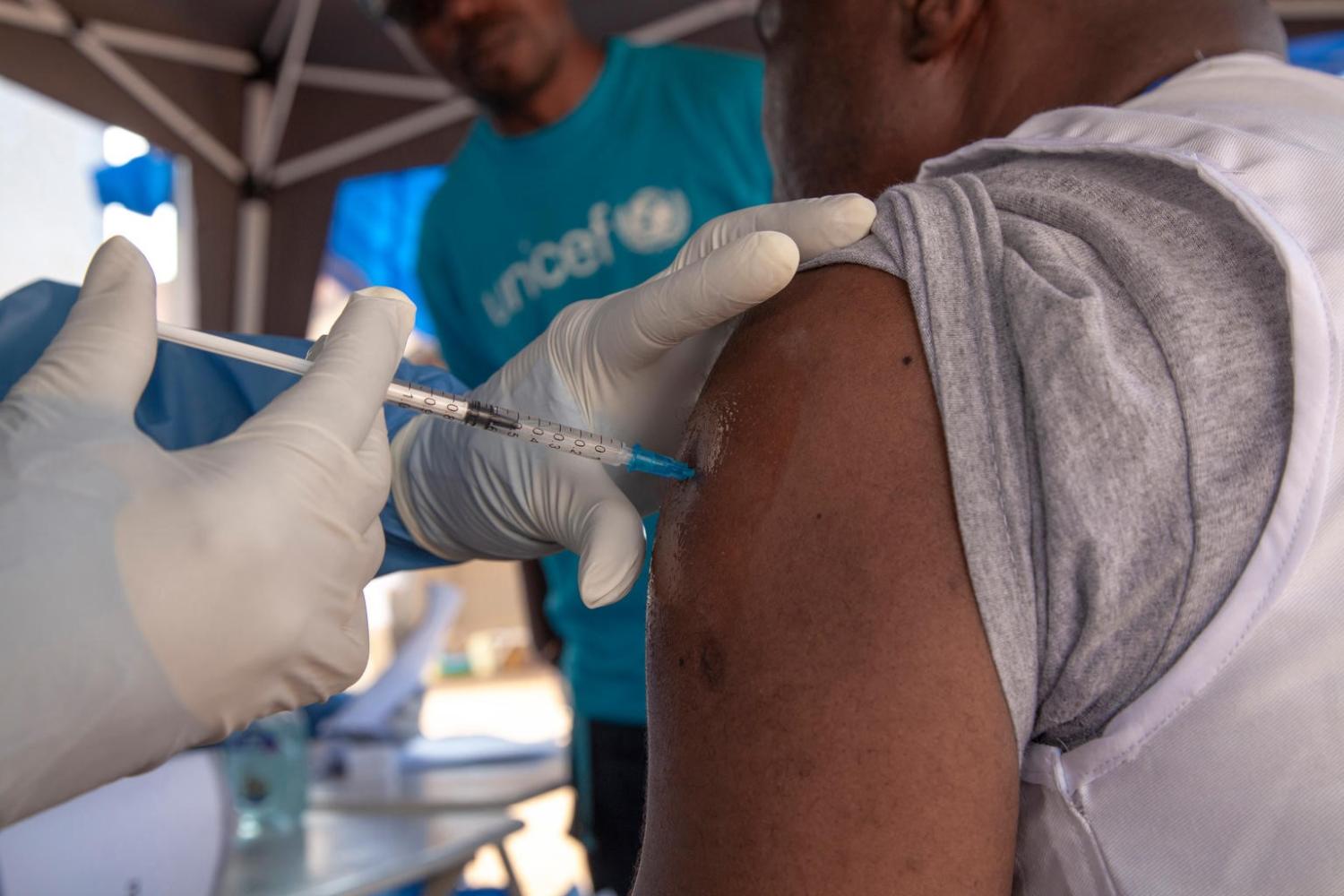
The World Health Organization (WHO) announced today that it has prequalified Merck's Ebola vaccine, a move that comes just 1 day after the European Commission granted full approval for VSV-EBOV, which is in use on a compassionate basis and under further study in the current Ebola outbreak in the Democratic Republic of the Congo (DRC).
According to a statement sent to journalists, the WHO said prequalification means the vaccine meets the WHO's quality, safety, and efficacy standards, paving the way for United Nations agencies and Gavi, the Vaccine Alliance, to buy the vaccine for use in at-risk countries.
Tedros Adhanom Ghebreyesus, PhD, the WHO's director-general, said in the statement that prequalification marks a historical step toward ensuring that the people who most need the vaccine are able to receive it. "Five years ago, we had no vaccine and no therapeutics for Ebola. With a prequalified vaccine and experimental therapeutics, Ebola is now preventable and treatable," he said.
Today's WHO announcement made good on Tedros's comments yesterday in the wake of the European Commission's full licensure announcement, the world's first for any Ebola vaccine. On Twitter, he said the WHO's prequalification was expected within days.
In mid-October, the European Medicines Agency (EMA) conditionally approved the vaccine under the Ervebo brand name for active immunization of people 18 years and older to protect against Ebola.
To speed the WHO prequalification process, the group reviewed the vaccine's safety and efficacy data as soon as they were available, and representatives from the WHO's prequalification team were on hand for the EMA's evaluation process to address program suitability for Africa's at-risk countries.
In today's announcement, the WHO said it is facilitating VSV-EBOV's licensing in at-risk countries and, with support from the EMA, has worked closely with many African nations to quickly approve the vaccine following the WHO's recommendation.
Step closer to global vaccine stockpile
In a statement welcoming yesterday's European Commission approval Seth Berkley, MD, Gavi's chief executive officer, said the vaccine has "huge potential" and has already been used to protect more than 250,000 people in the DRC and has the potential to make major Ebola outbreaks a thing of the past.
The European Commission approval paves the way for a Gavi-supported stockpile, he said. "It's also important to credit the unprecedented global effort from African countries that helped generate the evidence as well as Merck, WHO, donor governments, partners and regulatory agencies in making this authorisation happen."
At its next meeting in December, Gavi's board is expected to decide on a long-term Ebola vaccine program that would include creating a global stockpile, which it said hinges on the WHO's prequalification decisions for vaccines and the WHO vaccine advisory group's recommendations for their use.
Alongside the stockpile, the board will also consider, if recommended, future support for preventive vaccination of groups outside of outbreak settings, such as healthcare workers in high-risk countries.
Gavi and Merck have supported the stockpile of investigational Ebola vaccine, and Gavi has offered prepaid commitments to all Ebola vaccine manufacturers with products in phase 1 clinical trials to buy doses of vaccine when they are licensed and available. Gavi has also provided $15.1 million to the WHO to cover vaccination operational costs in the DRC and neighboring countries.
No new cases today
For the third day in a row, the DRC reported no new Ebola cases, keeping the total at 3,287, including 118 listed as probable, according to numbers reflected on the WHO's Ebola online dashboard. Health officials are still investigating 545 suspected cases.
Also, no new deaths were reported, keeping the fatality count at 2,192.
See also:
Nov 11 CIDRAP News story "European Commission grants final approval to Merck's Ebola vaccine"
Nov 12 Gavi statement
WHO online Ebola dashboard