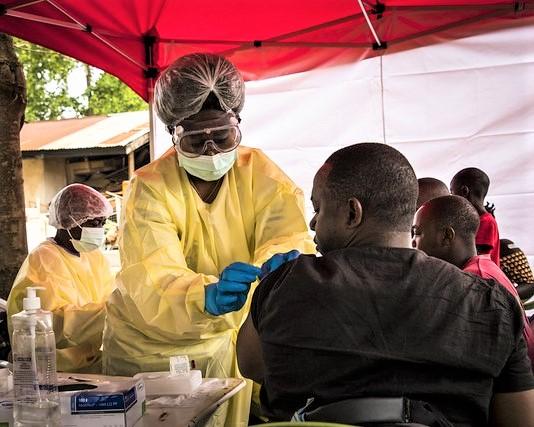
In an unusual statement surrounding a suspected Ebola death in Tanzania, the World Health Organization (WHO) on Sep 21 raised concerns about undiagnosed febrile illnesses in that country, which it said has withheld information about suspicious Ebola-like illness cases in violation of international health regulations.
In other key developments, the WHO today said the Democratic Republic of the Congo (DRC) has cleared the way for the use of a second experimental vaccine in the outbreak region, and Doctors Without Borders (MSF) called for an independent commission to manage vaccine stocks, saying more speedy and widespread use of the highly effective VSV-EBOV vaccine is needed to help cut the mortality rate.
Facts on suspected Tanzania cases unclear
The WHO said it has received unofficial reports on a number of unknown febrile illness cases in Tanzania through its regular surveillance process. They involve a person with suspected Ebola who died in Dar es Salaam, the country's former capital and biggest city, and contacts quarantined in various parts of Tanzania.
The WHO said an unofficial report on Sep 11 said that Ebola tests were positive for a patient with a suspected infection, and on the same day a second unofficial report said another suspected Ebola case in Mwanza (in the north of the country) tested negative for the virus. The following day, the WHO received another unofficial report of a death of a 27-year-old suspected patient who was hospitalized in Dar es Salaam, but there was no information on lab tests and results.
"Despite several requests, WHO did not receive further details of any of these cases from Tanzanian authorities," the WHO said.
A few days later, Tanzanian officials told the WHO that tests have confirmed no Ebola cases in the country and it is not considering secondary confirmation testing, a step routinely recommended by the WHO. Tanzanian officials told the WHO the two suspected cases tested negative by RT-PCR, but there was no information on differential diagnoses.
Then on Sep 19, the WHO received another report that a contact of the patient said to have initially tested positive is sick and hospitalized, prompting another denial from Tanzania that it has no Ebola cases and that there are no hospitalizations of suspected cases.
Rumors have been swirling over the past 2 weeks about suspected Ebola cases in Tanzania, which has never reported a case before. High-level health officials from the United States recently visited the Ebola outbreak region and neighboring countries, and Alex Azar, Health and Human Services (HHS) secretary, while speaking in Uganda urged Tanzania's government to share lab results regarding the case.
The US Centers for Disease Control and Prevention (CDC) announced on Sep 17 that CDC Director Robert Redfield, MD, visited Tanzania that day at Azar's request to meet with Tanzanian health officials and the CDC personnel who work closely with the government. The CDC statement said Redfield would focus on how the US can collaborate with Tanzania and support its efforts to prevent, detect, and respond to infectious diseases.
Information gaps hamper risk assessment
The WHO said it continues to reach out to Tanzania to verify rumors under International Health Regulations and has deployed a multidisciplinary rapid response team to the country to expand its response capacity in the WHO's country office. It added that the team is available to provide Tanzanian officials with technical assistance, if they request it.
The WHO said it needs clinical data, investigation findings, and information on contacts and lab tests to fully assess the risk posed by the event, which, if confirmed, would be Tanzania's first Ebola outbreak.
For now, given that presumptive positive patients traveled extensively within Tanzania, the national risk is high, the WHO said. And because of possible cross-border travel, population movements, and potential unknown transmission chains, the risk at the regional level is also high, it added.
It notes that Tanzania has been preparing for Ebola since May 2018, by establishing an Ebola treatment center in Dar es Salaam, as well as a public health emergency operation center. Three rapid response teams have been trained, a hotline for alerts has been activated, and sensitization efforts are underway. However, WHO added that an Ebola simulation exercise in August found areas for improvement.
Tanzanian officials have provisionally approved a VSV-EBOV vaccination protocol for immunizing frontline health workers, the WHO said, adding that it recently shipped personal protective equipment, logistics equipment, and supplies to support vaccination.
DRC green-lights use of second vaccine
Meanwhile, in DRC developments, government officials announced plans to use a second experimental vaccine, a two-dose regimen made by Johnson & Johnson, the WHO said today in a statement. The vaccine, known as Ad26.ZEBOV/MVA-BN, is a prime-boost product given 56 days apart and is designed to provide longer lasting protection against the virus.
In April, a WHO vaccine advisory group recommended adding Ad26.ZEBOV/MVA-BN as a way to increase supply and test the effectiveness of a second vaccine, but the country's former health minister pushed back against mounting international pressure to use it, because it could complicate vaccine messaging and post logistical challenges in delivering the second dose.
In its statement, the WHO said health officials will introduce the vaccine in the middle of October, targeting at-risk population in areas that don't have active Ebola transmission.
Matshidiso Moeti, MBBS, who directs the WHO's regional office for Africa, said, "The evaluation of the second Ebola vaccine will help ensure that we have potentially an additional tool to prevent the expansion of the outbreak and also a potential tool to protect populations before outbreaks hit areas at risk."
The WHO said at the current number of cases being reported, supplies of the current VSV-EBOV vaccine are sufficient. Merck, the vaccine's maker, has provided the WHO with 245,000 doses and built a stockpile of 190,000 doses that are ready to send to the DRC. Also, the WHO said Merck's goal is to release 650,000 doses over the next 6 to 18 months.
MSF calls for independent vaccine panel
In other vaccine developments, Doctors Without Borders (MSF) today called for an international, independent committee to transparently manage Ebola vaccine stocks and their use, saying not enough people have been vaccinated, partly because the WHO is strictly limiting the number of doses used in the field.
The MSF said despite the use of new therapies and vaccine in in the DRC's current outbreak, the mortality rate is about 67%, similar to that of West Africa's 2014-2016 outbreak when no treatments or vaccines were available.
Though a highly effective vaccine is available, the number of people vaccinated isn't enough, the group said. It said the pace of vaccination is too slow, and only a fraction of the eligible population is benefitting from the critical tool, it added.
One reason is the WHO's management of vaccine supplies, which MSF says is opaque, with strict limits on doses deployed to the field.
Natalie Roberts, MD, MSF emergency coordinator, said in the statement, "It's like giving firefighters a bucket of water to put out a fire, but only allowing them to use one cup of water a day."
MSF said the DRC's health ministry and the WHO have vaccinated nearly 220,000 people with VSV-EBOV, but the number is largely insufficient. Isabelle Defourny, MD, MSF's director of operations, said, "We think that upping the pace of vaccination is necessary and feasible: at least 2,000-2,500 people could be vaccinated each day, instead of 500 – 1,000 people as is currently the case."
She said WHO is restricting vaccine availability in the field and eligibility criteria for people to be vaccinated for reasons that aren't clear. "Even when it comes to frontline health workers – a known, easily reachable population – in a hotspot of the outbreak such as Beni, almost a third of them reported they have not been vaccinated," Defourny said.
MSF called for the urgent creation of an independent, international coordinating committee based on a 1997 group that helped manage limited vaccine supplies during a massive outbreaks of meningitis, cholera, and yellow fever vaccine.
Outbreak total rises by 11 cases
In the DRC, health officials reported 11 more Ebola cases over the weekend and through today, bringing the outbreak total to 3,168 cases. Authorities are still investigating 415 suspected cases.
Four more people died from their infections, lifting the fatality count to 2,115.
The DRC's multisector Ebola committee (CMRE) said in an update yesterday, which covers four of the most recent cases, that case locations include Komanda, Mambasa, and Mandima.
See also:
Sep 21 WHO statement
Sep 16 AP story on Azar transparency comments
Sep 17 CDC statement
Sep 23 WHO statement
Sep 23 MSF statement
WHO online Ebola dashboard
Sep 22 CRME report