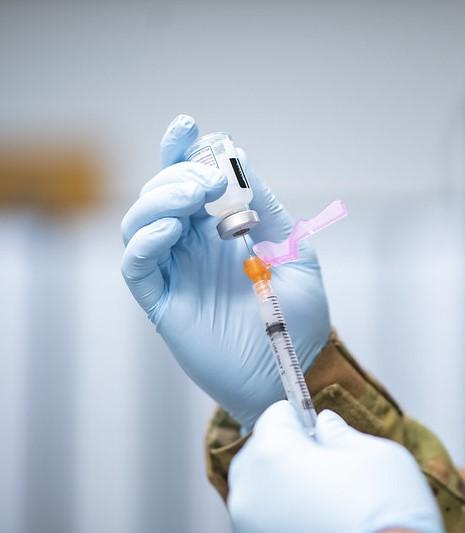
The Food and Drug Administration (FDA) as early as today may approve "mix and match" booster shots, meaning people can get a different COVID-19 vaccine from the one administered during their initial series if they need a booster.
Sources told the Washington Post the FDA could make the announcement at the same time it authorizes booster doses for some Moderna recipients, and all Johnson & Johnson (J&J) recipients.
The move is based on early data presented to the FDA late last week from the National Institutes of Health, as well as evidence from the European Union and United Kingdom, where mixed dosing has been used successfully.
The announcement will likely offer guidance to the 15 million Americans inoculated with the Johnson & Johnson (J&J) vaccine, which has proved less effective than either the Pfizer or Moderna vaccines. Though J&J recipients could choose to get a second dose of that vaccine, they could also choose to mix their initial adenovirus-based vaccine with an mRNA shot. This strategy, called heterologous prime-boosts, has been shown to be well-tolerated.
Currently, the FDA has unanimously approved a J&J booster, but later this week the Centers for Disease Control and Prevention (CDC) advisory group, ACIP (Advisory Committee on Immunization Practices), will meet to discuss booster shots for Moderna and J&J recipients.
CDC: Pfizer vaccine protective for kids 12-18
Today the CDC published a study in Morbidity and Mortality Weekly Report on adolescent recipients of the Pfizer/BioNTech vaccine, the only vaccine authorized for use in the United States for children ages 12 to 18.
The vaccine was 93% effective against hospitalization for the age-group, in a study that looked at hospitalizations in 16 states from June through September of this year, when the Delta (B1617.2) variant was the dominant strain of COVID-19.
A total of 179 hospitalized children were included in the study, only 3 of whom were vaccinated.
"In this real-world analysis, in which all case-patients were hospitalized, vaccination reduced the risk for COVID-19 hospitalization in persons aged 12–18 years by 93%," the authors concluded. "Moreover, 16% of patients hospitalized with COVID-19 had critical illness requiring life support; all were unvaccinated."
The findings of the study could guide federal regulars who will look at authorizing Pfizer for children ages 5 to 11 in the coming weeks.
15% of seniors have received a booster
The 7-day average of new daily COVID-19 cases is 83,341, with 1,631 daily deaths, according to the New York Times tracker. Yesterday, the tracker showed the same number of average cases in the United States, but a slightly lower average number of deaths, at 1,613.
The CDC COVID Data Tracker shows 57% of Americans have been fully vaccinated against COVID-19, 66% have received at least one dose of vaccine, and 5.6% of fully vaccinated people have received a booster dose.
So far, about 15% of people 65 and older have received a COVID-19 booster shot, according to a CNN analysis of CDC data.
Other US developments
- Seattle reported yesterday that 99% of its employees are in compliance with the mayor's COVID-19 vaccine mandate, according to the Associated Press.
- The head football coach at Washington State University was fired yesterday for refusing to get a COVID-19 vaccine as required by a mandate for state employees, NPR reported today. Four assistant coaches were also terminated.
- The average weekly positive rate among students in New York City's public schools is 0.25%, well under the city's rate of 2.43%, according to the New York Times. But experts say the city may not be testing enough.