Dr. Brosseau is a national expert on respiratory protection and infectious diseases and professor (retired), University of Illinois at Chicago.
____________________________________
Many experts in public health have, for very good reason, voiced frustration at the lack of science-based information they read regarding the ongoing COVID-19 pandemic. This is compounded by sometimes conflicting recommendations by the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC).
But by applying what we know about similar infectious diseases and pairing it with what the data show so far with this novel coronavirus and what common sense tells us, we can advise both healthcare professionals and the general public on what steps they can take to minimize their risk.
And it's OK to say that we're still gathering evidence.
An often ignored, yet important mode of transmission for infectious respiratory diseases—close-range aerosol transmission—needs to be part of the equation, and I'll detail the science on that later on. But, by taking lessons from recent research on similar aerosol-transmissible diseases, such as SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome)—both similarly caused by coronaviruses—and influenza, the following conclusions can be drawn.
Better communication is needed
Infection prevention, medical, and public health professionals should be communicating to everyone that the exact modes of transmission for SARS-CoV-2—the technical name of the virus that causes COVID-19—are unknown. There are no studies, yet, to support any particular mode of transmission over another.
The precautionary principle suggests we should approach this organism as we would any novel highly transmissible respiratory disease—as a contact, droplet and airborne disease, but with one important caveat: Short-range aerosol transmission is also a strong possibility. Taking lessons from the little we already know about COVID-19 as well as influenza, SARS, and MERS, all of which show many similarities to COVID-19, the precautionary approach suggests that we focus on preventing short-range aerosol transmission in both public and healthcare settings.
The approaches in each setting will differ, but the reasoning for these differences must be explained to avoid sending mixed messages that induce confusion, anger, and failure to follow recommendations.
We need to strategically protect health workers
Healthcare organizations should be protecting their workers and patients by following CDC guidelines.1 They should be rapidly triaging patients as they come in the door, rapidly separating and isolating those with respiratory symptoms, and ensuring all of their workers are fit-tested and have respirators. For aerosol-generating procedures, the CDC should be recommending respirators with higher levels of protection than an N95 filtering facepiece respirator (eg, a powered air-purifying respirator), but at this point, it does not.
In the face of supply shortages, the CDC last week changed its recommendations to allow the use of medical masks instead of respirators, saving the latter for aerosol-generating procedures.1 Healthcare organizations must return to using respirators for confirmed and suspected COVID-19 patients when supply chain problems are resolved. Requirements for airborne infection isolation rooms remain in place. Organizations are encouraged to designate entire units for COVID-19 patient care and develop re-use procedures for personal protective equipment.
All of these are reasonable modifications given the circumstances, but healthcare organizations should see them as neither CDC backing down from its original recommendations nor as permission to offer inadequate protection to healthcare workers when such equipment is available.
Healthcare organizations and public health agencies should consider the utility of reusable respirators, such as elastomeric respirators more commonly found in industrial settings. Such respirators may be in limited supply, but they offer some advantages, in that healthcare employees can be given an individual respirator for which they are responsible.
Healthcare organizations should also be considering source controls, such as cohorting suspected and confirmed patients, limiting the number of healthcare workers involved in care, using telemedicine, designating separate locations for triage and care, and deploying remote technologies, where possible.
In addition, public health and government agencies should be making every effort to support healthcare and other workers who protect the public. Transportation, safety, security, and infrastructure workers should have first priority for personal protective equipment and, if exposed, immediate coronavirus testing. Public health leaders should make every effort to prevent community transmission by, when appropriate, establishing social distancing measures.2
In addition to being clearer in its messaging about disease transmission, the CDC should be working hard to ensure there are enough testing kits and laboratories available, purchasing or providing personal protective equipment (including respirators), supporting public health agencies with thoughtful decision-making and policies, and insisting that workers who protect the public are themselves protected. The CDC should also insist that healthcare organizations follow the guidelines for airborne precautions while also encouraging the public to limit transmission by limiting contact.
What the public can do
The public should avoid crowded spaces, stay home when possible, prepare for a lengthy period at home in case of quarantine, and follow public health and government instructions.
Since medical mask s and respirators are most important for preventing the spread of aerosols from patients and protecting healthcare workers, the public should not be buying, hoarding, or stealing them. The public should not be wearing respirators at work, especially if they are experiencing respiratory symptoms, but instead staying home.
s and respirators are most important for preventing the spread of aerosols from patients and protecting healthcare workers, the public should not be buying, hoarding, or stealing them. The public should not be wearing respirators at work, especially if they are experiencing respiratory symptoms, but instead staying home.
And they should call ahead to a medical professional when having COVID-19 symptoms rather than add to already crowded waiting rooms.
Mixed messages about COVID-19 transmission
To date there is no direct research-based evidence describing exactly how SARS-CoV-2 is transmitted. Many sources say that COVID-19 is transmitted only by droplets and contact, but guidance from leading public health groups on transmission routes are inconsistent and conflicting.
(Droplet transmission is usually defined as "respiratory droplets carrying infectious pathogens [that] transmit infection when they travel directly from the respiratory tract of the infectious individual to susceptible mucosal surfaces of the recipient, generally over short distances, necessitating facial protection."3 Close contact involves hand transfer of surface contamination to mouth, nose or eyes, hand washing and gloves being common controls.)
The WHO says, "Based on the available evidence, the COVID-19 virus is transmitted between people through close contact and droplets, not by airborne transmission."4 The WHO derived its COVID-19 guidance from its MERS guidance,5 China's experience with COVID-19, and WHO experience with SARS and MERS.6
(Airborne transmission is defined as "dissemination of either airborne droplet nuclei or small particles in the respirable size range containing infectious agents that remain infective over time and distance."7 An important requirement of airborne transmission is that it can occur only at a long distance from the source, according to the CDC.8)
In risk communication guidelines for healthcare, however, the WHO states, "COVID-19 appears to spread most easily through close contact with an infected person. When someone who has COVID-19 coughs or sneezes, small droplets are released and, if you are too close, you can breathe in the virus" (emphasis added).9 But wait: Inhalation is not part of the traditional definition of droplet transmission.
For healthcare organizations, the CDC recommends airborne, in addition to standard (contact) and droplet precautions, for the care of COVID-19 suspected or confirmed patients.10
For the general public, the CDC describes SARS-CoV-2 transmission as primarily by droplets from coughs or sneezes, which "land in the mouths or noses of people who are nearby or possibly inhaled into the lungs" (emphasis added).11 But, again, inhalation is a new addition to the traditional definition of droplets. In contrast to its recommendations for healthcare, the CDC makes no mention of airborne transmission in public settings.
The CDC admits some possibility that COVID-19 may be transferred by hands to mouth, nose, or eyes from contaminated surfaces, but notes that "this is not thought to be the main way the virus spreads."11
The Chinese Center for Disease Control and Prevention, which has dealt with by far more COVID-19 cases than any other agency, says that COVID-19 transmission occurs primarily by respiratory droplets and close contact, with the "possibility of aerosol transmission in a relatively closed environment for a long time exposure to high concentrations of aerosols."12
Close-range aerosol transmission
Underlying the CDC and WHO statements about transmission is this: Inhalation of particles near the source may be an important mode of transmission.
Based on research now more than 70 years out of date, the infection control paradigm of contact, droplet, and airborne transmission fails to recognize inhalation of small airborne particles very close to an infectious source—ie, within 6 feet.13
Some everyday examples might help for illustration. Have you ever seen dust particles traveling through the air in a beam of light? Some of these eventually deposit on surfaces, but many remain airborne for long periods. Have you ever used hairspray or aerosolized cooking oil? Many of those droplets remain airborne nearby as you inhale particles and smell hairspray and cooking oil for several minutes.
The same thing happens when someone coughs or sneezes. Talking, breathing, coughing, and sneezing create an aerosol (a suspension of particles in the air) containing particles in a range of sizes, with viable infectious organisms present in both small and large particles.14-20
Infectious aerosols are inhalable
Contrary to popular belief, the larger particles (5 to 15 micrometers [µm]) will not immediately drop to the ground but will remain airborne for several minutes. Smaller particles (less than 5 µm) will remain in the air for many minutes or even hours.
All particles will immediately begin to evaporate (mucus contains a lot of water), which means the range of particle sizes will decrease overall. Smaller particles are more affected by diffusion than gravity, thus making them more likely to remain airborne. In the absence of air currents, airborne particles will disperse slowly throughout a space (see the figures below).
Figure 1. When an aerosol is initially emitted (time = 0), the particles are clustered near the source at location A. A person near the source (location B) may receive large-particle spray and inhale particles of all sizes.
Figures: Absolute Science Illustration
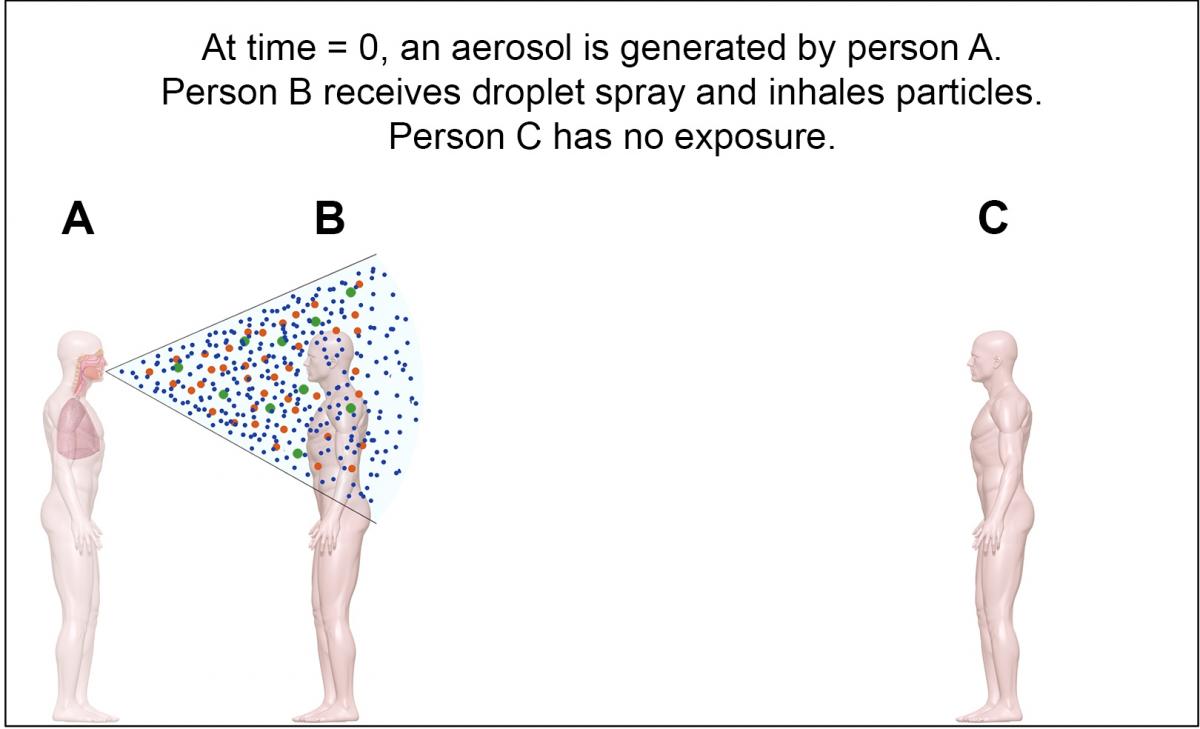
Figure 2. After some time (time = 1), the particles begin to disperse and larger particles begin to settle from the air. Person B will continue to inhale particles of all sizes.
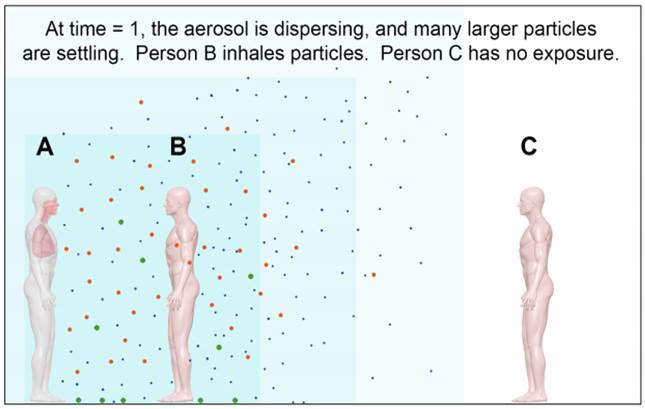
Figure 3. After more time (time = 2), the small particles are uniformly dispersed and more of the larger particles have settled from the air. Persons B and C will inhale particles that are generally smaller, have a smaller size range, and are at a lower concentration than at time = 0.
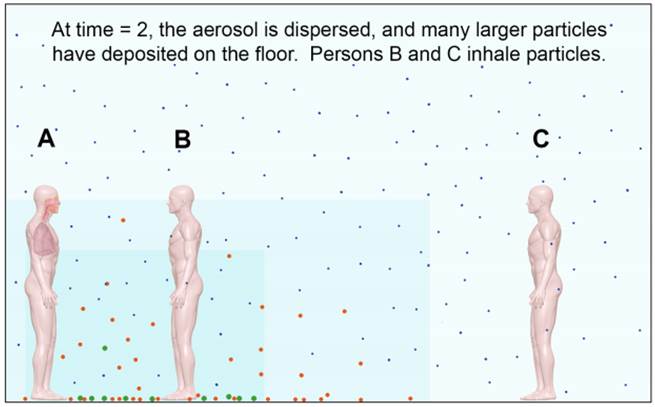
All of the particle sizes in a typical cough or sneeze aerosol are inhalable. The larger particles will deposit in the nose, while smaller particles deposit in the lungs, where cell receptors for many infectious respiratory viruses are typically located.
Likelihood of infection from close-range aerosols
Higher doses of infectious particles are more likely to result in infection and disease. Healthcare workers, whose work brings them close to more people with more severe symptoms in relatively enclosed spaces, are at more risk than the general public at being exposed to a dose of infectious particles that could lead to infection.
The Wuhan, China, experience supports the likelihood of close-range aerosol transmission. After initially receiving and treating COVID-19 patients in the existing healthcare system and experiencing healthcare worker infections, China deployed a tiered hospital model very similar to that used for Ebola patients in the United States.21 Patients with critical or severe symptoms were moved into designated wards or hospitals while those with mild symptoms were cohorted in temporary hospitals in repurposed buildings. Healthcare workers wore full protection, including a gown, head-covering, N95 filtering facepiece respirators, eye protection, and gloves.22
A recent non-peer-reviewed report from Germany supports aerosol transmission of COVID-19 at close range.23 (ref Woelfel et al, 2020). Throat and nasal swabs from nine patients in Germany with mild symptoms found virus RNA in upper respiratory system tissues, with the highest amounts of virus shedding in the first 5 days as symptoms were developing. Live virus was found in 17% of swab samples and 83% of sputum samples in the first week but not after day 8. Different genotypes found in a paired throat swab and sputum sample from one patient suggest that replication may occur in the throat independently from the lung. These findings differ from sampling in SARS patients, in whom nasal swabs were much less likely to be positive, live virus isolation from throat swabs was very rare, viral loads were 1,000 times lower, and build-up took 7 to 10 days after symptom onset. All urine and blood samples in the German patients were negative.
The authors conclude that SARS-CoV-2 may be more efficient at transmitting than SARS-CoV through active shedding from the upper respiratory tract as symptoms are developing. Later on, COVID-19 is more like SARS, with replication in the lower respiratory tract. They conclude: "Our initial results suggest that measures to contain viral spread should aim at droplet-, rather than fomite-based transmission." As discussed above, these findings also support the possibility of close-range aerosol transmission.23
As well, another recent non-peer-reviewed article demonstrates that COVID-19 aerosols can be present in healthcare settings.24 Air sampling followed by analysis for RNA concentrations found no SARS-CoV-2 in the intensive care unit, critical care unit, and patient areas of a Wuhan hospital designated for patients with severe COVID-19 symptoms, thought to be due to negative-pressure ventilation and high air exchange rates. Low concentrations (6 copies/m3) were found in medical staff areas.
Aerosols were found in two separate size ranges: 0.25 to 1 µm, and larger than 2.5 µm. In a temporary Wuhan hospital fashioned from an indoor sports stadium for cohorting and treating patients with mild symptoms, high RNA concentrations were found in rooms used for removal of protective clothing (18 to 42 copies/m3), with the highest concentrations found in 0.25- to 0.5-µm particles, thought to result from particle release from contaminated clothing.
High RNA concentrations (19 copies/m3) were measured in patient toilets in both hospitals. Toilet flushing is well-known as a source of aerosols.25 The Wuhan study offers no insights into the viability of these aerosols, however, as live virus was not assessed.
Droplet transmission likely less important than thought
Droplet transmission is probably much less important for most respiratory infectious diseases than is short-range aerosol transmission by inhalation. Aerosol particles are not all large, and they do not all immediately fall to the ground. It is rare for coughs or sneezes to be propelled into nearby mouths or noses. The eyes, however, may be a portal of entry for some infectious organisms, such as influenza viruses.26,27
What aerosol transmission with other diseases can tell us
The traditional definition of airborne transmission—long-range inhalation of droplet nuclei—arises simply because some organisms are hardier than others. Tuberculosis and measles (classic examples of airborne respiratory diseases) remain viable in air for long periods. Viability dissipates with time, not distance. Therefore, diseases that are considered "airborne" must also be capable of transmitting disease by inhalation of aerosols near the source.
Close-range aerosol transmission
An increasing number of studies with animals and in human settings indicate that close-range aerosol transmission by inhalation is important for influenza.28-31 SARS and MERS demonstrate increased transmission in healthcare settings, especially to healthcare workers near aerosol-generating procedures. Tellier et al30 concluded that airborne transmission is likely for these two coronaviruses based on epidemiologic investigations, human respiratory sampling, and lower respiratory tract receptors for MERS.
Viability in air
A very recent study found that SARS-CoV-2 aerosols remain viable for up to 3 hours,32, which is similar to the viability of SARS-CoV in air33 and MERS-CoV.34,35 This is adequate time for exposure, inhalation, and infection to occur both near and far from a source.
Transmission in healthcare settings
SARS-CoV and MERS-CoV exhibit high levels of transmission in healthcare settings, in particular during aerosol-generating procedures. More than half of those contracting SARS during the 2003 pandemic were healthcare workers.
As of early February 2020, more than 3,000 healthcare workers were believed to have contracted COVID-19 in China, and at least 6 died.37
These data, along with recent US reports of healthcare worker infections in long-term care facilities and employees on cruise ships, are suggestive of both short- and long-range aerosol transmission in healthcare and other workplace settings.
Contact transmission of influenza, SARS, and MERS
The possibility of contact transmission and the utility of hand washing for any organism should be informed by scientific data that support biological plausibility (eg, receptors for the organism in the nose, mouth, or eyes) or demonstrate transmission in relevant animal species or humans.
Data support influenza transmission to the eyes in ferrets.26,27 The effectiveness of hand hygiene in community settings is minimal.38 Few data are available on contact transmission for SARS or MERS, although it seems unlikely if receptors are located primarily in the lower respiratory tract.30
Data are thus only somewhat suggestive of contact as a mode of transmission for COVID-19 in community settings and align with CDC advice that it is not the most important mode.
We'll navigate this pandemic guided by science
It is possible to get through this pandemic together with thoughtful, consistent, science-based and open communication and decision-making. It is possible to communicate uncertainty about the underlying science, apply the precautionary approach where uncertainty exists, and implement well-reasoned decisions about how best to limit the dissemination of COVID-19.
It is unfortunate we are not as prepared as we could have been, given the many years we've been planning for pandemic influenza. But rather than criticizing past decisions, I am hopeful we can move forward together in a way that ensures our healthcare and other high-priority workforces have the highest degree of protection possible. And perhaps we'll make better decisions going forward, once this pandemic is over.
References
- CDC. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings.
- CDC. Coronavirus disease 2019 (COVID-19): schools, workplaces & community locations. Updated Mar 15, 2020
- CDC. Infection control: isolation precautions.
- WHO. Advice on the use of masks in the community, during home care and in healthcare settings in the context of the novel coronavirus (2019-nCoV) outbreak. Jan 29, 2020
- WHO. Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: interim guidance. Updated October 2019
- WHO. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19): interim guidance. Feb 27, 2020
- CDC. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. 2007
- CDC. Infection control: how infections spread.
- WHO. The COVID-19 risk communication package for healthcare facilities. Updated Mar 10, 2020
- CDC. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. Updated Mar 10, 2020
- CDC. Coronavirus disease 2019 (COVID-19): how COVID-19 spreads.
- Chinese Center for Disease Control and Prevention. General questions: COVID-19 prevention and control.
- Gralton J, Tovey E, McLaws ML, et al. The role of particle size in aerosolised pathogen transmission: a review. J Infect 2011 Jan;62(1):1-13
- Yang S, Lee GWM, Chen CM, et al. The size and concentration of droplets generated by coughing in human subjects. J Aerosol Med 2007 winter;20(4):484-94
- Milton DK, Fabian MP, Cowling BJ, et al. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 2013 Mar 7;9(3):e1003205
- Lindsley WG, Pearce TA, Hudnall JB, et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg 2012 Dec 11;9(7):443-9
- Lindsley WG, Blachere FM, Thewlis RE, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLOS One 2010 Nov 30;5(11):e15100
- Noti JD, Lindsley WG, Blachere FM, et al. Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clin Infect Dis 2012 Jun 1;54(11):1569-77
- Blachere FM, Lindsley WG, Pearce TA, et al. Measurement of airborne influenza virus in a hospital emergency department. Clin Infect Dis 2009 Feb;8(4):438-40
- Yan J, Grantham M, Pantelic J, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA 2018 Jan 30;115(5):1081-6
- CDC. Interim guidance for U.S. hospital preparedness for patients under investigation (PUIs) or with confirmed Ebola virus disease (EVD): a framework for a tiered approach.
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Feb 25, 2020
- Woelfel R, Corman VM, Guggemos W, et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv 2020 Mar 8
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. bioRxiv 2020 Mar 10
- Johnson DL, Mead, KR, Lynch RA, et al. Lifting the lid on toilet plume aerosol: a literature review with suggestions for future research. Am J Infect Control 2013 Mar;41(3):254-58
- Belser JA, Gustin KM, Katz JM, et al. Influenza virus infectivity and virulence following ocular-only aerosol inoculation of ferrets. J Virol 2014 Sep;88(17):9647-54
- Belser JA, Lash RR, Garg S, et al. The eyes have it: influenza virus infection beyond the respiratory tract. Lancet Infect Dis 2018 Jul;18(7):e220-7
- Zhou J, Wei J, Choy KT, et al. Defining the sizes of airborne particles that mediate influenza transmission in ferrets. Proc Natl Acad Sci USA 2018 Mar 6;115(10):E2386-92
- Mermel LA. The great influenza centennial—what have we learned about the epidemiology and prevention of transmission?Clin Microbiol Infect 2018 Dec;24(12):1227-8
- Tellier R, Yuguo Li Y, Cowling BJ, at al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019 Jan 31;19(1):101
- Cowling BJ, Ip DK, Fan, VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun 2013 Jun 4;4:1935
- van Doremalen N, Bushmaker T, Morris D, et al. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. medRxiv 2020 Mar 13
- Pyankov OV, Pyankova OG, Agranovski IE. Inactivation of airborne influenza virus in the ambient air. J Aerosol Sci 2012 Nov;53:21-8
- Pyankov OV, Bodnev SA, Pyankova OG, et al. Survival of aerosolized coronavirus in the ambient air. J Aerosol Sci 2018 Jan;115:158-63
- Van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill 2013 Sep 19;18(38):20590.
- MacIntyre CR, Chughtai AA, Rahman B, et al. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influenza Other Respir Viruses 2017;11(6):511-7
- Zhou P, Huang Z. Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol 2020 (published online Mar 5)
- Moncion K, Young K, Tunis M, et al. Effectiveness of hand hygiene practices in preventing influenza virus infection in the community setting: a systematic review. Can Commun Dis Rep 2019 Jan 3;45(1):12-23
Other resources
Brosseau L, Ann RL. N95 respirators and surgical masks. NIOSH Science Blog. Oct 14, 2009
Jones R, Brosseau L. Aerosol transmission of infectious disease. J Occup Environ Med 2015 May;57(5):501-8
NIOSH. Workplace safety and health topics: respirators
Shaffer R, Cichowicz JK, Chew G, et al. Non-occupational uses of respiratory protection – what public health organizations and users need to know. NIOSH Science Blog. Jan 4, 2018
Sietsema M, Radonovich L, Hearl FJ, et al. A control banding framework for protecting the US workforce from aerosol transmissible infectious disease outbreaks with high public health consequences. Health Secur 2019 Apr 19;17(2):124-32






















