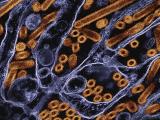
Nov 15, 2006 (CIDRAP News) – Scientists say they have developed an inexpensive "gene chip" test that can quickly identify a variety of influenza A viruses, including H5N1, and is less apt to be confused by viral mutations than other tests are.
A research team from the University of Colorado in Boulder and the Centers for Disease Control and Prevention (CDC) developed the test and will report their results in the Dec 15 issue of Analytical Chemistry. The test involves a microarray of genetic material—short sequences of known RNA deposited on a microscope slide.
The team says the test has a key advantage over other available tests in that it is based on a single gene segment that mutates less often than the gene segments used in other tests, meaning it may not have to be updated as frequently as viruses evolve.
Researchers examined the test's ability to identify 24 H5N1 viral isolates and distinguish them from seven non-H5N1 isolates. They found that the "MChip" provided complete information about virus type and subtype for 21 of the 24 H5N1 isolates and gave no false-positives, according to a news release from the National Institute of Allergy and Infectious Diseases (NIAID), which funded the research.
The samples were collected between 2003 and 2006 from people and animals in places as widely separated as Vietnam, Nigeria, Indonesia, and Kazakhstan. Six of the human isolates were taken from an Indonesian family in which human-to-human transmission was suspected.
Analysis of 47 samples consisting of influenza A viruses (subtypes H5N1, H3N2, and H1N1) and negative controls revealed the test's clinical sensitivity was 97% and its clinical specificity was 100%, according to the journal article.
Nancy J. Cox, PhD, director of the CDC's influenza division and a coauthor of the report, said in the press release that the new technology, when commercially available, could revolutionize the way laboratories test for influenza.
"The MChip could enable more scientists and physicians, possibly even those working in remote places, to more quickly test for H5N1 and to accurately identify the specific strain and its features," Cox said. "This would greatly increase our ability to learn more about the viruses causing illnesses and take the best steps to respond."
Kathy L. Rowlen, PhD, a University of Colorado professor who led the research team, said in the news release that the MChip is an improvement over its predecessor, the FluChip. The latter uses three influenza genes—hemagglutinin (HA), neuraminidase (NA), and matrix (M)—whereas the MChip uses only the M gene.
Rowlen said HA and NA are difficult to use in flu diagnostic tests because they mutate constantly. "The M segment is much less of a moving target than the HA and NA gene. We believe that a test based on this relatively unchanging gene segment will be more robust," she said.
The MChip displays results as a pattern of fluorescent spots that are automatically interpreted by artificial neural network software designed to eliminate human error. Rowlen said the software component will make the test easier to use in the field.
Rowlen told CIDRAP News that a private company is negotiating with the University of Colorado to manufacture the test for commercial use. She projected that the deal will be completed within the next few months, but she wasn't sure when the test would be submitted for approval to the Food and Drug Administration (FDA). "It depends on how much time and energy the company can pour into it."
If approved by the FDA, the MChip system could be in great demand in a flu pandemic. Rowlen said foreign supply-chain disruptions in the event of a pandemic would probably not seriously affect MChip production. She said most of the raw materials used to make the test, such as glass slides and reverse transcription–polymerase chain reaction reagents, are supplied by US companies. The cost of the raw materials is about $10.
Dawson E, Moore CL, Dankbar DM, et al. Identification of A/H5N1 influenza viruses using a single gene diagnostic microarray. Anal Chem 2006 Dec 15, in press
See also:
Nov 13 NIAID press release on MChip
http://www.niaid.nih.gov/news/newsreleases/2006/Pages/mchip.aspx
Dawson ED, Moore CL, Smagala JA, et al. MChip: a tool for influenza surveillance. Anal Chem 2006 Nov 15;78(22):7610-5 [Abstract]
Aug 29 CIDRAP News story on FluChip test: "New test may enable more labs to subtype flu viruses"