Advances in testing have prompted new guidelines for the diagnosis of latent or active tuberculosis (TB) in adults and children.
The guidelines, published today in Clinical Infectious Diseases, were developed by a task force supported by the Infectious Diseases Society of America (IDSA), the American Thoracic Society (ATS), and the Centers for Disease Control and Prevention (CDC). They are the first new guidelines on TB diagnosis released in 17 years. Their intended purpose is to define high- and low-risk patient populations based on the results of epidemiologic studies, provide diagnostic recommendations that lead to beneficial treatments, and describe a classification scheme for TB that is based on pathogenesis.
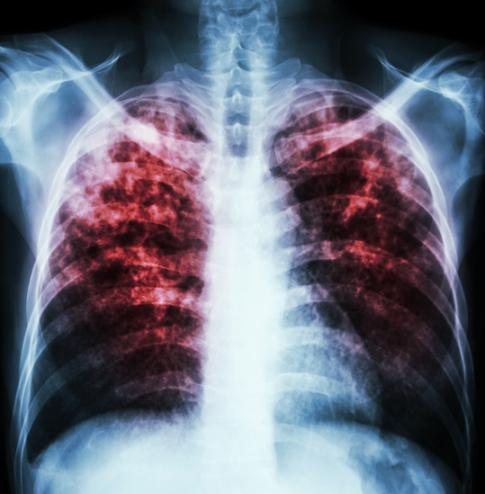
TB disease, which is caused by the Mycobacterium tuberculosis bacterium, is one of the major causes of morbidity and mortality worldwide. The World Health Organization (WHO) estimates that 8.6 million cases of TB occurred in 2014, and 1.5 million died from the disease. The disease is spread through the air when infected people cough, sneeze, or even speak.
The authors of the guideline note, however, that because the disease far less prevalent in the United States (only 9,557 active cases were reported in 2015), doctors may overlook TB as a possible diagnosis. This is further complicated by the fact that while some infected individuals develop signs and symptoms of the disease (active TB), people with latent TB infection (LTBI) have the bacteria in their body but show no clinical evidence of the disease.
"Even though TB disease is not common in this country, it's important that doctors remember it's still around, and that they should test patients when appropriate," lead author David Lewinsohn, MD, PhD, a professor of medicine at Oregon Health & Science University, said in an IDSA press release.
According to IDSA, as many as 13 million Americans may have latent TB.
Three high-risk groups
The first recommendation regards diagnostic testing for patients suspected of having LBTI, and it focuses on three groups considered at high risk of being infected: People who have household contact or have been exposed to a TB patient, immigrants from high-risk countries, and resident or workers in high-risk settings, such as prisons.
For these individuals, the guidelines recommend doctors perform an interferon-gamma release assay (IGRA) rather than a tuberculin skin test (TST). IGRAs are newer tests that are considered more effective because they measure the cellular immune response in the blood. They also can be performed in a single visit, whereas TSTs require two visits. But the authors note that a TST is an acceptable alternative when an IGRA is not available, too costly, or too burdensome.
The aim of testing patients who have suspected LTBI, the authors write, is to identify those who will benefit from prophylactic therapy, which can prevent progression to active TB and in doing so reduce the risk of patients transmitting the disease to others (people with LTBI can't spread the bacteria). Without treatment, patients with LTBI have a 4% to 6% lifetime risk of developing TB disease.
"Without the application of improved diagnosis and effective treatment for LTBI, new cases of TB will develop from within this group, which is therefore a major focus for the control and elimination of tuberculosis," the authors write.
Testing for active TB, MDR-TB
Because IGRA and TST provide evidence of infection only and cannot distinguish between active and latent TB, the guidelines recommend that physicians rule out active TB before starting treatment for LTBI, since treatment for active TB and LBTI differs.
If a patient shows symptoms of TB disease—including fevers, night sweats, weight loss, and coughing—and chest x-rays indicate active TB, the guidelines recommend that a series of sputum tests be performed for confirmation of the disease—especially in high-risk individuals, such as HIV patients and those who live with someone with TB. These tests include smears, cultures, and a nucleic acid amplification test (NAAT).
NAATs are a form of molecular diagnostic testing that can provide quicker, more specific results than smears and cultures, but the authors note that they are appropriate only as an adjunct to those tests. Use of NAATs for TB diagnosis has only recently become standard practice.
The guidelines also suggest that rapid molecular drug susceptibility testing for the antibiotic rifampin should be performed on patients who have tested positive on either the smear or NAAT test and are considered at risk for multidrug-resistant (MDR) TB, which has emerged over the past two decades and is becoming a growing problem. The WHO estimates that 480,000 people worldwide developed MDR-TB in 2014.
The authors say the guidelines are aimed at clinicians in high-resource countries with low incidence of TB disease and LTBI but are not intended to impose a standard of care.
See also:
Dec 8 Clin Infect Dis TB diagnosis guidelines
Dec 8 IDSA press release