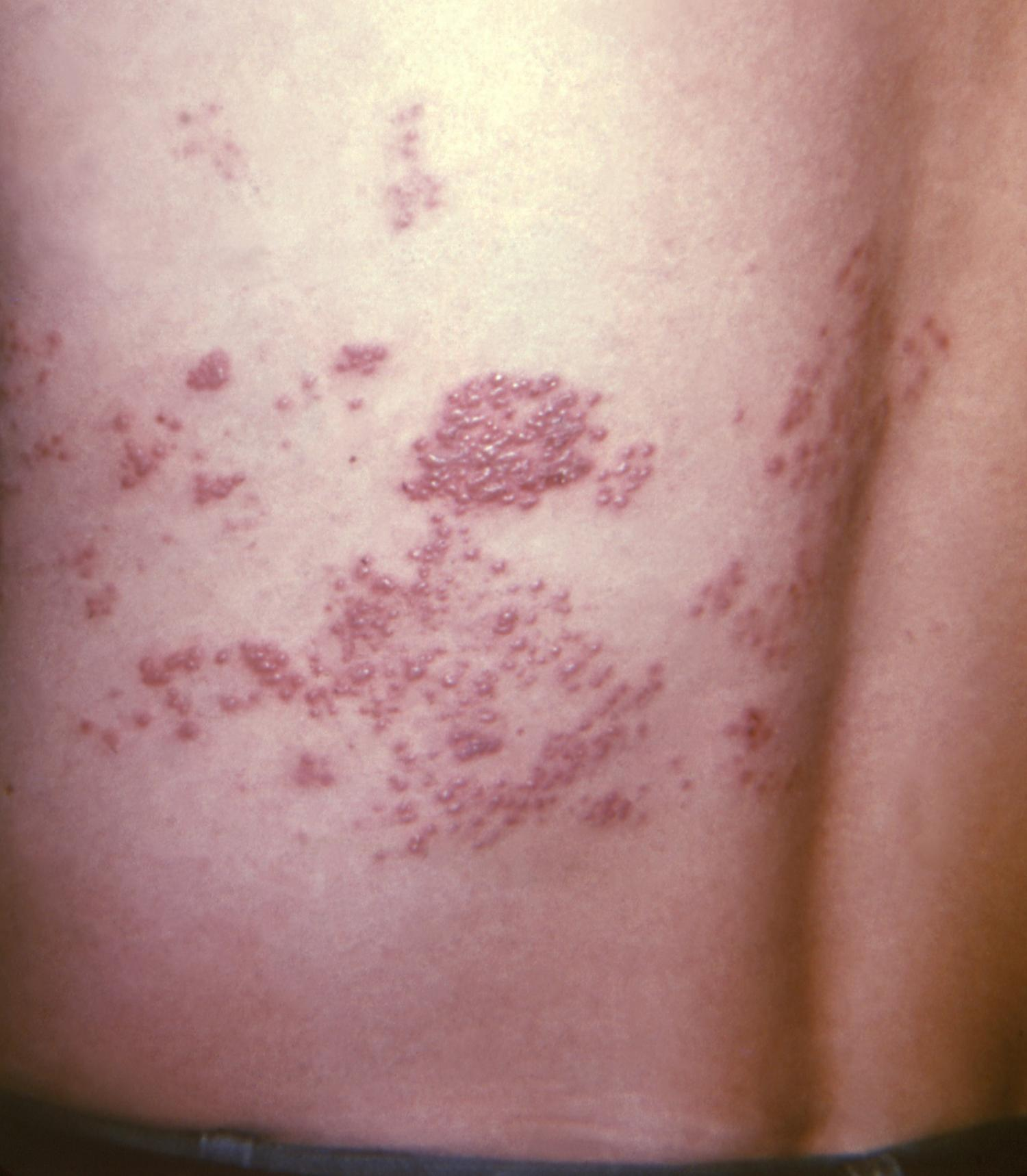
Two doses of the adjuvanted recombinant shingles vaccine (Shingrix) are an estimated 74% effective against herpes zoster infection and 84% effective against postherpetic neuralgia (PHN) in US adults ages 50 and older, according to interim results from a real-world study published in Clinical Infectious Diseases.
Shingles is a painful rash caused by reactivation of the varicella-zoster virus, which causes chickenpox. PHN, or nerve pain that lingers after the shingles rash resolves, occurs in 10% to 18% of shingles patients.
Researchers from Kaiser Permanente Southern California (KPSC) and shingles vaccine maker GSK matched older KPSC patients who received one or two vaccine doses from April 2018 to December 2020 in a 1:4 ratio with unvaccinated participants, with 2 years of follow-up. A total of 102,766 participants received two doses, and 192,984 received one dose.
The median patient age was 68 years, 59.0% were women, 57.1% were White, 17.8% were Hispanic, and 46.7% had previously received the now-discontinued live zoster vaccine.
Protection stable throughout follow-up
Rates of herpes zoster per 1,000 person-years were 9.2 in unvaccinated and 2.6 in two-dose vaccinees. The incidence of PHN per 1,000 person-years was 0.5 in unvaccinated and 0.1 in two-dose recipients.
These findings underscore the importance of adhering to the recommended vaccination schedule.
The adjusted vaccine effectiveness (aVE) of two shingles vaccine doses was 73.9% (95% confidence interval [CI], 71.8% to 75.8%) against herpes zoster infection. aVE was 83.7% (95% CI, 75.1% to 89.3%) against PHN. VE against both conditions was stable for at least 4 years after vaccination.
One dose of vaccine was 60.3% (95% CI, 56.4% to 63.9%) against herpes zoster and 45.6% (11.4% to 66.6%) against PHN.
VE against herpes zoster was 61.7% in patients with weakened immune systems, compared with 74.9% in those with intact immune systems. Similarly, VE against PHN was 44.5% in immunocompromised participants, versus 90.8% in healthy patients.
The study authors said they plan to follow participants until December 2030. "These findings underscore the importance of adhering to the recommended vaccination schedule," they wrote.
.jpg)












