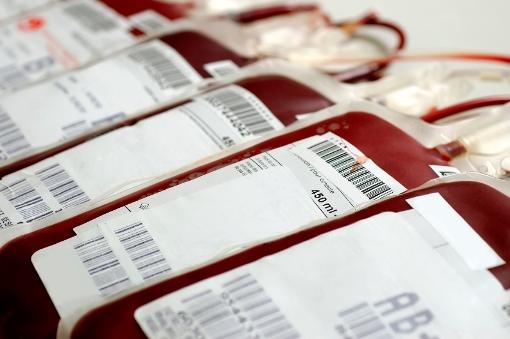
Based on Florida's investigation into the first possible locally acquired Zika virus cases in the continental United States, the US Food and Drug Administration (FDA) yesterday asked blood establishments in the two affected neighboring counties to immediately stop collecting blood.
In other developments, Zika infection numbers in Puerto Rico continue to rise at a fast clip, along with further increases in US travelers, including several pregnant women.
Testing, pathogen inactivation in blood
The blood collection step, which affects Broward and Miami-Dade counties, is a temporary measure to protect the blood supply until the blood groups are able to screen each unit of blood for Zika RNA or implement approved or investigational pathogen inactivation technology, the FDA said in its statement yesterday.
The Florida Department of Health (Florida Health) is investigating four suspected locally acquired Zika cases, two in each of the counties. In an update today, Florida Health reported two more travel-linked cases, both of them in pregnant women. Overall, the state has recorded 328 travel-related Zika cases, plus 55 more in pregnant women.
Last week when Florida reported its first suspected local case, OneBlood, which provides blood services throughout Florida and parts of Georgia and South Carolina, said it would start testing donated blood for Zika virus on Aug 1 using a new test that was granted emergency use authorization.
Puerto Rico Zika surge continues
In its updated Zika cases numbers for last week, the US Centers for Disease Control and Prevention (CDC) today reported 914 more infections in US territories, most in Puerto Rico. So far, 4,729 have been reported.
In US states, the number of travel-linked Zika cases also climbed at a steady pace, growing by 254 from the previous week to a total of 1,657.
In its update on pregnant women infected with Zika virus, the CDC reported 33 more cases in the states and 44 more in US territories, putting the totals at 433 and 422, respectively.
One more Zika-related birth defect was reported, in a baby born in a US state. Overall, the states have now reported 13 congenital Zika cases in newborns, along with 6 pregnancy losses related to Zika virus. The number in the US territories remained at 1, which reflects a pregnancy loss.
More Caribbean locations affected
In its weekly update on disease developments today, the World Health Organization (WHO) said one more country and one more territory have reported local Zika cases: the independent state of Antiqua and Barbuda and the British overseas territories Turks and Caicos.
One more country, Paraguay, has reported Zika-liked microcephaly cases, raising the total number of affected nations to 14, the WHO said. It said two lab-confirmed cases have been reported.
In Guinea-Bissau, where a joint mission is under way to investigate local cases and assess preparedness, one more sample has tested positive for Zika virus and 22 additional samples have been collected for testing, the WHO said.
Genetic sequencing tests results are still pending on four recent Zika samples to determine if infections there reflect further spread of the Americas strain into another part of Africa or if the cases involve the African strain that has been on the continent for decades.
Research developments
- Inovio this week announced that the first study volunteer in its phase 1 trial has received a dose of its Zika DNA vaccine. In a Jul 26 statement it said the trial, which has been cleared by US and Canadian regulators, will take place at clinical sites in Miami, Philadelphia, and Quebec City. It will involve 40 healthy adults and will gauge the safety and immunogenicity of the vaccine administered with Inovio's proprietary intradermal DNA vaccine delivery device. The company is developing the vaccine, called GLS-5700, with GeneOne Life Science and academic groups in the United States and Canada.
- In findings that run counter to two recent reports, blood and urine tests on 61 Brazilians who were assessed for Zika virus during the acute illness phase found a higher detection rate in plasma than in urine. Reporting in Eurosurveillance today, Brazilian and German researchers based their findings on tests done within the first 5 days of symptom onset. Among the patients, 46 were positive on blood tests and 37 had positive urine tests, a difference that the team said wasn't statistically significant. Both samples were positive in 28 patients, and viral loads were similar for both. Earlier studies suggested that the virus lingered longer and in higher levels in urine. The team said factors specific to the Brazilian population might explain the difference, and they recommended testing of both blood and urine during acute infection.
See also:
Jul 27 FDA press release
Jul 28 Florida Health daily Zika update
Jul 28 CDC update on Zika cases in US states and territories
Jul 28 CDC update on Zika infections in pregnant women
Jul 28 CDC update on Zika-related pregnancy outcomes
Jul 28 WHO Zika situation report