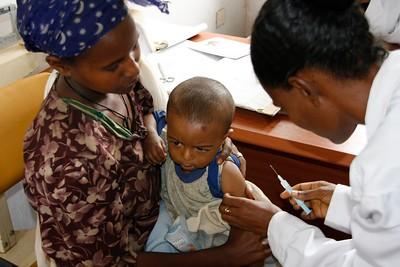
A phase 3 randomized controlled trial confirms that the R21/Matrix-M malaria vaccine is 67% to 75% effective against the mosquito-borne illness in young children in four African countries.
Study data, published yesterday in The Lancet, also confirm a good safety profile for the vaccine, which costs only $2 to $4 per dose, per the World Health Organization (WHO).
A University of Oxford–led research team randomly assigned 4,800 children aged 5 months to 3 years in Burkina Faso, Kenya, Mali, and Tanzania to receive either the malaria vaccine or a rabies control vaccine from April 2021 to January 2022. Follow-up was 1 year.
A total of 3,103 vaccine recipients and 1,541 controls were included in a modified per-protocol analysis; 51.9% were boys, and 48.1% were girls. The vaccine was given in three doses 4 weeks apart, followed by a booster 12 months after the third dose. Half of the children were enrolled at two sites with seasonal malaria transmission, and half were recruited from standard sites with perennial malaria spread.
Highest VE in kids aged 5 to 17 months
Vaccine effectiveness (VE) at 1 year was 75% (95% confidence interval [CI], 71% to 79%) at seasonal sites and 68% (95% CI, 61% to 74%) at standard sites for time to first malaria infection. VE against multiple infections was 75% (95% CI, 71% to 78%) at seasonal sites and 67% (95% CI, 59% to 73%) at standard sites.
Mild waning occurred over 1 year at similar rates at both site types. The investigators noted a rate reduction of 868 (95% CI, 762 to 974) cases per 1,000 children-years at seasonal sites and 296 (95% CI, 231 to 362) at standard sites.
Children aged 5 to 17 months had the highest VE regarding time to first clinical malaria episode at seasonal (79% [95% CI, 73% to 84%) and standard (75% [95% CI, 65% to 83%) sites. This age-group also had significantly greater immune responses than those aged 18 to 36 months.
The vaccine was well tolerated, with 18.6% of 1,615 participants experiencing injection-site pain and 46.7% of 1,615 experiencing fever. The number of adverse events of special interest and serious adverse events didn't differ significantly between the two vaccine groups. No vaccine-related deaths were reported.
The WHO recommended the vaccine in October 2023.
Low cost, fast scale-up
"This low-cost, high-efficacy vaccine is already licensed by several African countries, and recently received a WHO policy recommendation and prequalification, offering large-scale supply to help reduce the great burden of malaria in sub-Saharan Africa," the study authors wrote.
The vaccine was developed by the University of Oxford and vaccine maker Serum Institute of India, which is ready to roll out the first 25 million doses in the next 3 or 4 months, according to a university news release. Up to 200 million doses are predicted to be available in the coming years.
"The continued high efficacy of this new vaccine in field trials is very encouraging, and consistent with the high efficacy and excellent durability observed in a smaller four-year phase IIb trial," chief investigator Adrian Hill, MD, PhD, of the University of Oxford, said in the release.
This low-cost, high-efficacy vaccine is already licensed by several African countries, and recently received a WHO policy recommendation and prequalification, offering large-scale supply to help reduce the great burden of malaria in sub-Saharan Africa.
In a related commentary, Vasee Moorthy, BMBCh, PhD, of the WHO, and colleagues said that R21/Matrix-M will augment the 18 million doses of the RTS,S/AS01 malaria vaccine expected to be available this year and next (annual demand is expected to be 80 million to 100 million doses).
"Two key advantages of R21/Matrix-M are the price, which is expected to be substantially lower than the current RTS,S/AS01 price, and the much higher near-term supply capability," they wrote.
Moorthy and colleagues urged the integration of malaria vaccines into WHO-recommended high-impact measures for infection control in areas of moderate to high transmission. "Effective malaria control depends on the strategic deployment of a mix of partially effective interventions, including vaccines," they concluded. "Importantly, financing for malaria vaccines must be additive to and not replacing existing WHO-recommended malaria control measures if the unacceptable burden of malaria mortality is to be curtailed."