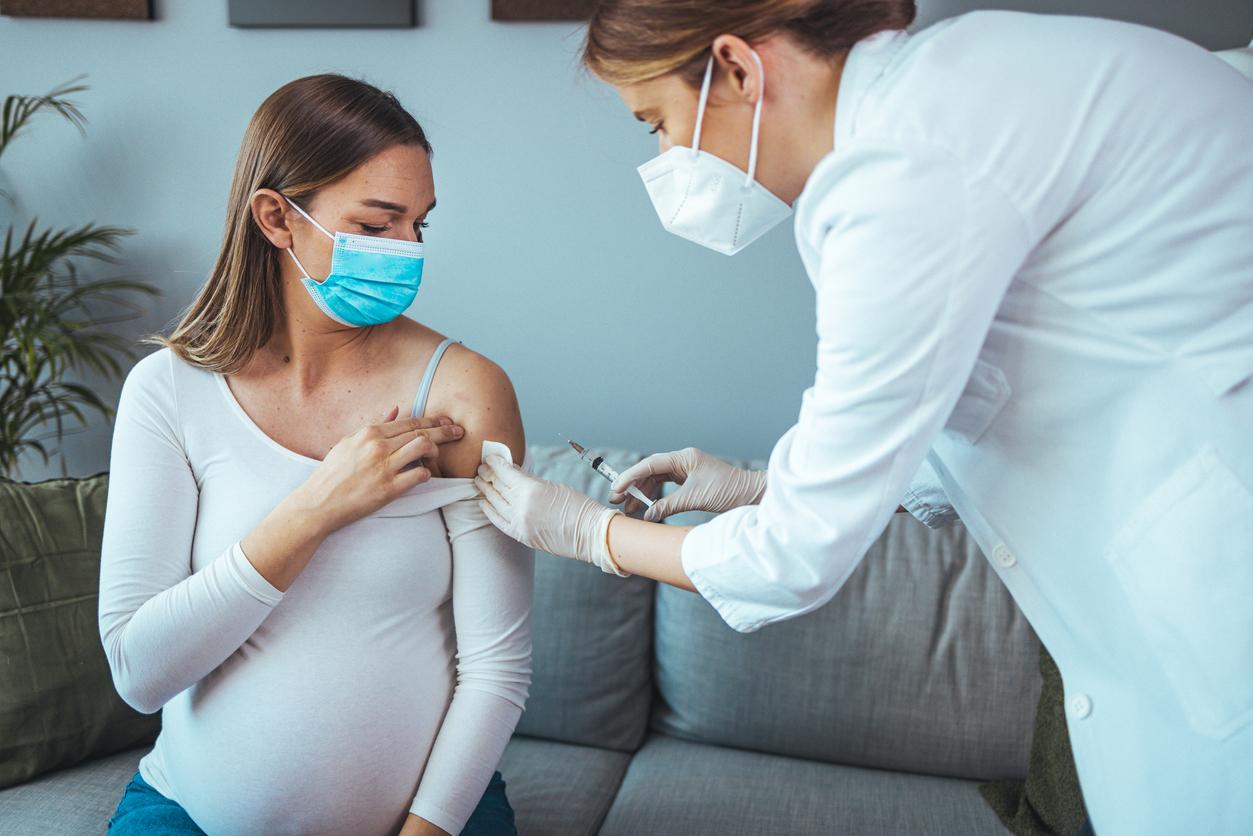
The vaccine advisory group to the Centers for Disease Control and Prevention (CDC) today approved a recommendation for pregnant women to receive Pfizer's respiratory syncytial virus (RSV) vaccine as a way to protect newborns, a group at high risk for serious complications.
The Advisory Committee on Immunization Practices (ACIP) recommended that pregnant women between 32 and 36 weeks gestation receive the vaccine as a single intramuscular dose seasonally to prevent infections in infants. The vote passed by an 11 to 1 margin.
Complex issues with rollout of new monoclonal vaccine for babies
During today's discussion, the group grappled with complicated issues regarding practical challenges of the rollout of a fourth vaccine recommended for pregnant women. Members also raised issues on how to balance RSV vaccination for mothers, usually managed by obstetricians, with the arrival of the new monoclonal RSV vaccine (nirsevimab-alip, also known as Beyfortus) for babies, which would be handled by pediatricians.
Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, raised the issue about gaps in vaccine registries, which could leave pediatric providers inadequate information to gauge whether an infant should receive the new monoclonal antibody vaccine. She also said families could face unexpected medical expenses if they face coverage issues for the RSV vaccine and the monoclonal antibody vaccine. She urged medical societies to improve communication about the new options for cutting the RSV burden in infants.
Other ACIP members acknowledged that more education for providers, both obstetric and pediatric, is needed to help navigate the use of both new RSV products. Jamie Loehr, MD, a family doctor in Ithaca, New York, suggested that Pfizer provide doctors with vaccine cards to give their vaccinated pregnant patients to help them and pediatricians track the mother's RSV vaccination status.
Other members raised the point that having two strategies for curbing RSV in infants is an exciting development. Katherine Poehling, MD, MPH, a pediatrics professor at Wake Forest School of Medicine, said about 80% of babies admitted to intensive care units for severe RSV have no underlying health conditions. She added that the discussions are complex, but making good use of the new treatments is feasible if everyone does their part.
Patsy Stinchfield, RN, MS, CPNP, an ACIP liaison committee member who was on the RSV vaccine work group, said forming the recommendation was very complex and challenging. "This is one of those times when we have to focus, that the vaccine is safe, effective and feasible and that practice will follow policy." She added that work will continue on practice barriers while advancing a good product.
Group votes to add vaccine to VFC program for younger moms
In a second vote today, ACIP recommended by the same voting margin to add the RSV vaccine for pregnant women younger than age 19 to the Vaccines for Children (VFC) program, a federally funded program that provides free vaccine to children who don't have health insurance or can't afford the cost.
Pregnant women are the second group recommended to receive the RSV vaccine. In late June, the CDC endorsed the use of two newly approved RSV vaccines in adults ages 60 and older, based on shared decision making with clinicians. However, today's ACIP recommendation applies only to Pfizer's vaccine, called Abrysvo. In mid-August, the Food and Drug Administration (FDA) approved the use of Pfizer's vaccine in pregnant women between 32 and 36 weeks gestation as a way to protect infants as old as 6 months.
Soon after the vote, the CDC issued a statement via e-mail that said its director Mandy Cohen, MD, MPH, accepted the recommendation, and the group added that the vaccine is available in some US locations and that it expects availability to increase in the weeks ahead.
"I encourage parents to talk to their doctors about how to protect their little ones against serious RSV illness, using either a vaccine given during pregnancy, or an RSV immunization given to your baby after birth," Cohen said.